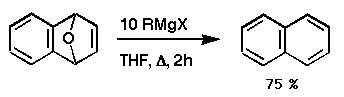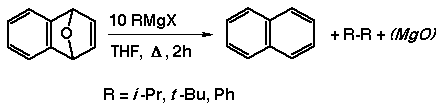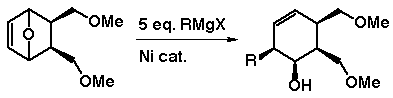Title
Introduction
Other Methods
Precedents
Why we were doing this chemistry
Known Methodology
Discovery
Mechanism
Summary of Results
Our efforts to understand the mechanism began with a study of the
effect of benzyne on the endoxide, since we initally believed benzyne
to be responsible through possible radical interaction with the
endoxide. Benzyne was involved, but not through interaction with the
substrate!
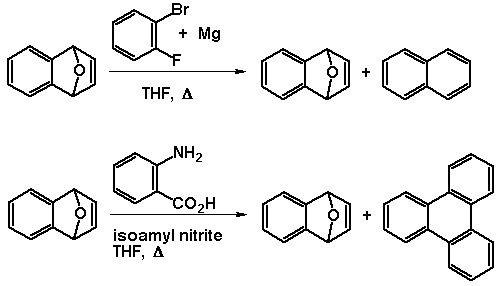
Benzyne, by itself was shown not to be the cause of this
transformation by the following experiment. Benzyne generated by the
decomposition of anthranilic acid by isoamylnitrite in the presence
of endoxide does not give naphthalene, but instead returns the
endoxide and triphenylene. No naphthalene could be detected.
We thus began to consider that either the Grignard reagent, the
Mg metal itself, through loss of MgO, or a metal-aromatic charge
transfer complex might be involved.
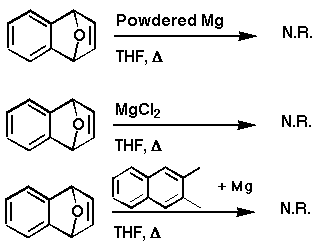
We further narrowed our search by systematically eliminating
these possibilities. Freshly purchased, powdered Mg in the presence
of 1,4-dihydro-1,4-epoxynaphthalene in refluxing THF, over several
days, failed to produce any naphthalene. This turned out to be
deceptive, because we were later to find that the Mg simply wasn't in
an active enough form!
Second, magnesium dihalides were applied as mimics for the
possible action of MgBrF which we thought might be setting up a
complex with the heteroatom of the endoxide, eventually resulting in
its removal. These experiments did not result in the formation of
naphthalene.
Lastly, an e- charge transfer mechanism was
eliminated by refluxing the endoxide shown with
2,3-dimethylnaphthalene and powdered Mg. There was no reaction. At
the same time, we had found that refluxing the endoxides in the
presence of excess Grignard reagents gave naphthalene, but still
believed that this result was proceeding by a different mechanism
then that of the benzyne chemistry.
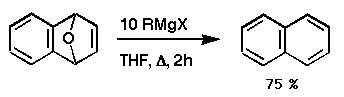
Reinvestigation of the original experiment, being more careful
this time not to lose any volatile products, revealed a great deal
about the mechanism.
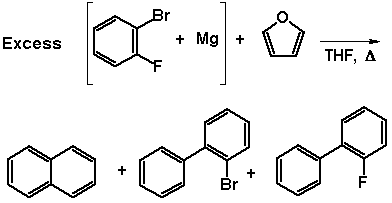
The GC/MS trace of the non-purified reactions (simply quenched,
so as not to lose any volatile products), revealed 2-bromo- and
2-fluoro-biphenyl.
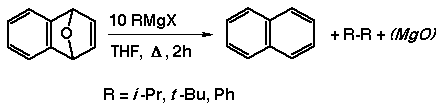
Reinvestigation of the Grignard reactions in this way also
revealed the presence of, in every case, the bis-organic Wurtz-like
product of the given Grignard. We confirmed our GC/MS determinations
with proton and carbon magnetic resonance experiments.
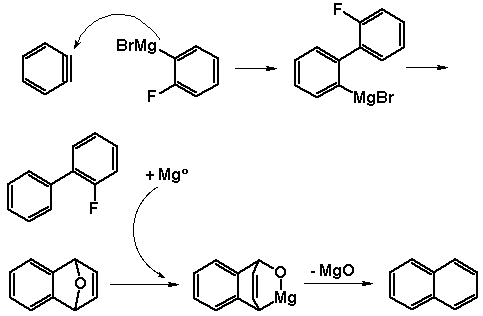
At this point, we realized that the mechanisms of both the
Grignard initiated and 'benzyne' involved route were probably
similar. Deoxygenation of the endoxide was probably accomplished by
the formation of a highly activated Mg species generated. We believe
this to be explained through the action of the Schlenk equilibrium on
Grignard reagents.
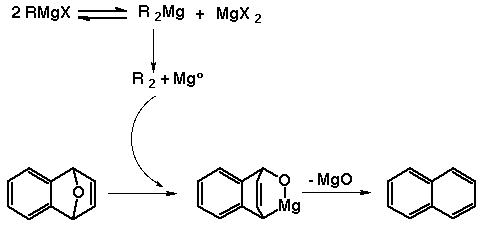
We believe that the Schlenk equilibrium must not proceed very
readily to Mg(0) and organic coupling products very much, because the
formation of the coupling product acts as a sink. It could be
imagined that only 2 equivalents of the Grignard to endoxide would be
needed to complete this reaction, but it doesn't do so, at least not
in a reasonable amount of time. Excess Grignard reagent seems to be
needed to ensure that enough Mg(0) is formed to carry out the
reaction, the residual being lost on workup.
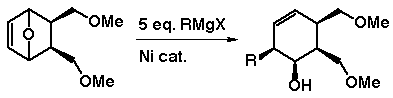
Interestingly, the reactivity of endoxides to Grignard reagents
observed here is very different to the reactivity of non-benzo-fused
1,4-epoxides with Grignard reagents. (Lautens, M.; Ma, S. J. Org.
Chem. 1996, 61, 7246.)
Back to top of page.