Title
Introduction
Other Methods
Precedents
Why we were doing this
chemistry
Known Methodology
Discovery
Mechanism
Summary of Results
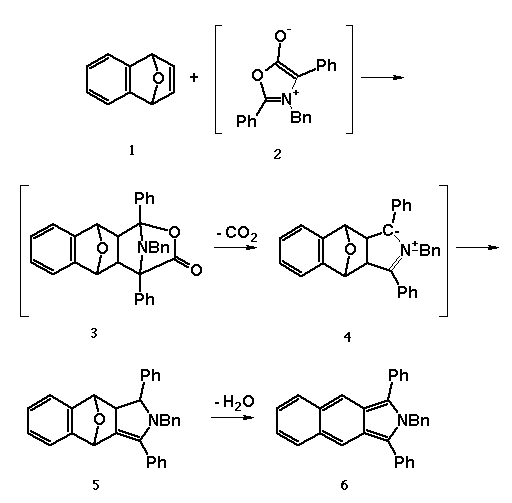
We made our discovery while preparing the starting endoxide, 1,
shown below, for a novel synthesis of the benz[f]isoindole skeleton.
We've found that the endoxide reacts with mesoionic munchnones, 2,
prepared by the in situ dehydration of the corresponding amino acid,
to give a 1,3-dipolar cycloadduct, 3. This adduct readily loses
CO2 to yeild a second 1,3-dipolar cycloadduct, 4, which
then undergoes a proton shift rather then react intermolecularly to
compound 5. The stable intermediate thus formed is then easily
elaborated to the benz[f]isoindole, 6, by dehydration.