| [Related articles/posters: 028 011 016 ] |
Reactivity Studies of [Pd2(u-Br)2(PBut3)2].Structural characterisation of phosphine cyclometallated cluster [Pd4(u-Br)3(u-CO)2(PBut3)3]
D. Michael P. Mingos,a* Ramón Vilar,a Mary McPartlinb and Ian Scowenb.
Department of Chemistry, Imperial College of Science, Technology and Medicine, South Kensington, London SW7 2AY, U.K.
Department of Chemistry, University of North London, Holloway Road, London, U.K.
Abstract
Recently considerable interest has been shown in palladium(I) dimers as possible intermediates in catalytic systems for organic transforations. In this paper we report some reactivity studies for the recently prepared dimers [Pd2(u-X)2(PBut3)2] (X=Br, 1, I, 2). The reactions of 1 and 2 with CO, CNXyl, H2, C2H2 and [Co2(CO)8] have been studied and interesting products have been obtained. Particularly interesting are the reaction between the dimers and CO. When the 1 is treated with CO the two new cluster componds [Pd4(u-Br)3(u-CO)2(PBut3)3] (3) and [Pd6(u-Br)4(u-CO)4(PBut3)4] (4) are obtained. The X-ray crystal structure of the tetranuclear cluster 3 is reported showing interesting structural features.
Introduction
Recently considerable interest has been shown in palladium(I) dimers as possible intermediates in catalytic systems for organic transformations. Several compounds of this type containing a palladium-palladium bond have been previously synthesised and their reactivities studied.1,2 Interestingly, the simple halogen bridged dimers 1 and 2 had not been described until recently.
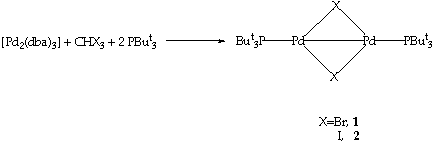
SCHEME 1
In a previous comunication3 we established that the reaction between [Pd2(dba)3] (dba=PhCHCHC(O)CHCHPh), PBut3 and the halocarbons CRX3 (X=Br, I) leads to the formation of the PdI-PdI dimers [Pd2(u-X)2(PBut3)2]. These dimers represent interesting precursors for the formation of high nuclearity clusters. On the other hand, their unsaturated electronic state, suggests that they may readily react with organic fragments to form organometallic compounds. In this paper we report a detailed study of their reactions with CO, H2, CNXyl and C2H2. In the reaction with CO the novel tetranuclear cluster [Pd4(u-Br)3(u-CO)2(But2PCMe2CH2)(PBut3)2] has been obtained and structurally characterised.
Results and Discussion
Reaction with CO: The addition of ligands to a pre-formed cluster can lead to a simple substitution reaction. However, sometimes the process is more complicated and cluster aggregation occurs.4 It has been previously observed5 that the reaction of some palladium clusters with CO leads to higher nuclearity compounds. In order to study this possibility, the reactions between the palladium dimers [Pd2(u-X)2(PBut3)2] (X=Br, I) and CO have been studied.
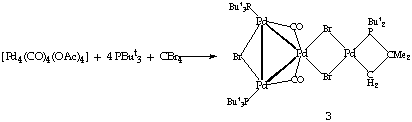
When CO was bubbled through a solution of [Pd2(u-Br)2(PBut3)2] in toluene an immediate colour change from green to dark orange was observed. The reaction mixture was analysed through 31P-{1H} NMR spectroscopy which showed the formation of two different products. By careful recrystallisation these two products were separated and characterised as [Pd4(u-Br)3(u-CO)2(But2PCMe2CH2)(PBut3)2] (3) and [Pd6(u-Br)4(u-CO)4(PBut3)4] (4) (see Scheme 2).

SCHEME 2
[Pd4(u-Br)3(u-CO)2(But2PCMe2CH2)(PBut3)2] (3) was extracted with hexane from the crude product and recrystallised from a toluene/hexane mixture at 4[ring]C. This orange product was characterised on the basis of 31P-{1H} NMR, IR, FAB-MS and elemental analyses (CLICK HERE FOR EXPERIMENTAL DETAILS). A single-crystal X-Ray analysis of compound 3 showed that it is a tetramer based on a palladium-triangle (see Figure 1) with an additional spike.
FIGURE 1 (Crystal Structure)
From the molecular structure, it is possible to see that there are two different phosphine environments: a cyclometallated PBut3 (phosphine bonded to Pd(4)) and two equivalent PBut3 coordinated to Pd(1) and Pd(3). The cyclometallated phosphine forms a four member ring with the palladium atoms in the spike of the structure. The Pd-Pd bond lengths cover a range from 2.702(1) to 3.072(1) Å. The longest Pd-Pd distance is between Pd(3) and Pd(4). This bond is bridged by two bromide ligands and is longer than the equivalent distance (2.628(2) Å) in the dimer 1.3 The CO ligands bridge the bonds Pd(1)-Pd(3) and Pd(2)-Pd(3) and the average Pd-CO distance is 1.966(1)Å. It is interesting to note that this structure resembles half of [Pd6(u-Br)4(u-CO)4(PBut3)4] (4)6 in which the two palladium triangles are bridged by two bromide ligands. It is possible that cyclometallation of the phosphine in structure 3 stops the aggregation process at the tetranuclear stage.
Interestingly cluster 3 has also been obtained as the major product of the reaction of [Pd4(OAc)4(PBut3)4] with PBut3 and CBr4 (CLICK HERE FOR EXPERIMENTAL DETAILS).
SCHEME 3
The second major product in the reaction between 1 and CO was isolated and purified by recrystallisation of the residual solid from an acetone/hexane mixture (CLICK HERE FOR EXPERIMENTAL DETAILS). This compound was formulated as [Pd6(u-Br)4(u-CO)4(PBut3)4]. The 31P-{1H} NMR spectrum showed a singlet at 87.1 ppm indicating the presence of only one phosphine environment. The IR showed a very strong band at 1879 cm-1 that can be assigned to a bridging CO. Elemental analyses for C and H showed that this compound has Pd/Br/CO/PBut3 ratio of 3/2/2/2 that is consistent with the proposed formulation. The same compound, i.e. [Pd6(u-Br)4(u-CO)4(PBut3)4], has been recently prepared (and structurally chararacterised) using a different route.6
When the dimer [Pd2(u-I)2(PBut3)2] was reacted with CO, an immediate change in colour from purple to dark red was observed. However, if the solution was not kept under a CO atmosphere the product readily released CO giving back the starting material. This reversible reaction was monitored by 31P-{1H} NMR and solution IR spectroscopy. When the reaction mixture was kept under a CO atmosphere and ethanol was added, after 24 hrs at 4[ring]C, a dark purple solid precipitated. The elemental analyses, together with the 31P-{1H} NMR and IR data, are consistent with the formulation [Pd6(u-CO)4(u-I)4(PBut3)4] (5) (CLICK HERE FOR EXPERIMENTAL DETAILS).
Reaction with CNXyl: Isocyanides behave in many respects like CO and can exhibit terminal or bridging coordination modes. In order to study the possible aggregation to high nuclearity clusters, the reactions between the dimers [Pd2(u-X)2(PBut3)2] (X=Br, I) and CNXyl were studied. When a solution of [Pd2(u-Br)2(PBut3)2] in toluene was treated with four equivalents of CNXyl, an immediate change of colour from green to orange was observed. The reaction was stirred for 2 hr and the 31P-{1H} NMR was measured. The 31P-{1H} NMR spectrum showed only one signal al 63.0 ppm (chemical shift for the free phosphine) suggesting that all the phosphines had been replaced by the CNXyl groups. Hexane was added to the reaction mixture and a dark yellow crystalline solid precipitated. On the basis of the IR, FAB-MS and elemental analyses, the product obtained was formulated as [Pd2Br2(CNXyl)4] . Analogous results were obtained when the dimer [Pd2(u-I)2(PBut3)2] was reacted with CNXyl. The resulting product was formulated on the basis of IR, FAB-MS and elemental analyses as [Pd2I2(CNXyl)4] CLICK HERE FOR EXPERIMENTAL DETAILS).
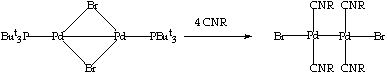
SCHEME 4
This type of compounds have been previously prepared from [Pd(dba)2], [PdCl2(C6H5CN)2] and CNR by Retting and Maitlis.7 The X-Ray crystal structures for [Pd2I2(CNMe)4] and [Pd2Cl2(CNBut)4] have been reported by Yamamoto8 and Balch9 respectively.
Reaction with H2: Palladium and platinum hydrido compounds have been implicated in several catalytic reactions.10 Thus, the synthesis of palladium-hydrido compounds is especially interesting. Very few dimers or clusters of palladium with hydrido groups coordinated to the metal core have been prepared.11 With this in mind, the reactions between the dimers and hydrogen were studied. When H2 was bubbled through a solution of [Pd2(u-Br)2(PBut3)2] in toluene for 15 minutes the original green colour of the solution disappeared giving a pale almost colourless solution and a black precipitate (palladium black). The solution was filtered, the solvent removed under reduced pressure and the colourless solid recrystallised from hexane at 4[ring]C overnight. The crystals obtained were identified on the basis of 31P-{1H} and 1H NMR as the compound trans-[PdHBr(PBut3)2]. The 31P-{1H} NMR spectrum of this compound showed a singlet at 84.0 ppm while the 1H NMR spectrum showed a triplet at -15.5 ppm suggesting the formation of a hydrido complex (the analogous hydrido compound trans-[PdHCl(PBut3)2] has been previously reported12 and shows a triplet in 1H NMR spectrum at -16.5 ppm and a singlet in 31P-{1H} NMR spectrum at 79.7 ppm). The splitting of the signal into a triplet, is due to the coupling between the hydrido group and the two chemically equivalent and trans phosphines in the compound. When the same reaction was repeated in the presence of two equivalents of PBut3 palladium black was no longer formed. Two signals in the 31P-{1H} NMR were observed: a singlet at 84.0 ppm due to the hydrido complex and a singlet at 85.5 ppm, which could be assigned to [Pd(PBut3)2].13
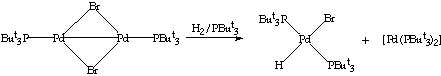
SCHEME 5
When H2 was bubbled through a solution of [Pd2(u-I)2(PBut3)2] in toluene, no reaction was observed. Even after several hours no changes in the original 31P-{1H} and 1H NMR spectra were observed.
Reaction with C2H2: The direct insertion of acetylenes into metal-metal bonds, has interesting catalytic implications. Representative examples of this type of reaction in palladium chemistry involve the insertion of alkynes into the Pd-Pd bond of dimers with formula [Pd2(dpm)2X2] (dpm=bis(diphenylphosphinomethane).14, 15 In these reactions compounds with a Pd-(CR)=(CR)-Pd moiety was isolated. These species have been implicated in the catalytic cyclotrimerisation of acetylene.14 In order to study this type of reaction in our systems, the dimers 1 and 2 were reacted with acetylene.
When C2H2 was bubbled through a solution of 1 in toluene, a dark precipitate and an orange solution were obtained. The insoluble solid was characterised on the basis of IR and elemental analysis as polyacetylene. The original dimer 1 is not present at the end of the reaction indicating that it acts only as a precursor for the catalysis. We are currently investigating the formulation of the palladium product resulting from this reaction. Interestingly, when this reaction was studied with the dimer [Pd2(u-I)2(PBut3)2], polyacetylene was also obtained but in this case, the original dimer was recovered after several hours of reaction. 31P-NMR indicated that the only phosphorous-containing product in solution was the original dimer 2. This suggests that 2 functions as a genuine catalyst.
Conclusions
The dimers [Pd2(u-X)2(PBut3)2] (X=Br, I) have proved to be very versatile precursors for different reactions. Firstly they function as [Pd(PBut3)X]-fragment sources for the synthesis of cluster compounds and this has been clearly exemplified by the syntheses of clusters 3 and 4. These dimers have also been used recently to prepare the bimetallic clusters [CoPd3(u3-X)(u-CO)3(u3-CO)(CO)(PBut3)3] (X=Br, I).6 We are currently studying the reactions of these dimers with a variety of alternative reagents to get a wide range of cluster compounds with the [Pd(PBut3)X] as a fundamental unit building block.
The second type of reaction that we are interested in, is the activation of small molecules between the two palladium centres. The reaction of the dimers with hydrogen, unfortunately, either did not react when X=I or resulted in the break down of the dimers (when of X=Br). We are currently investigating the possible catalytic implications of this reaction. The polymerisation of acetylene using 1 or 2 as catalysts, opens a wide area of possible study.
Acknowledgements
We thank CONACYT and ORS for scholarships to R.V. and BP plc for endowing D.M.P.M.'s chair.
References
[1] S. Ogoshi, K. Tsutsumi, M. Ooi and H. Kurosawa, J. Am. Chem. Soc., 1995, 117, 10415; T. Murahashi, N. Kanehisha, Y. Kai, T. Otani and H. Kurosawa,J. Chem. Soc., Chem. Commun., 1996, 825; H. Kurosawa, K. Hirako, S. Natsume, S. Ogoshi, N. Kanehisa and Y. Kai, Organometallics, 1996, 15, 2089.
[2] W. Lin, S.R. Wilson and G.S. Girolami, Inorg. Chem., 1994, 33, 2265; H. Felkin and G.K. Turner, J. Organomet. Chem., 1977, 129, 429; For a review: H. Werner, Adv. Organomet. Chem., 1981, 19, 155.
[3] R. Vilar, D.M.P. Mingos and C.J. Cardin, J. Chem. Soc., Dalton Trans., 1996, 4313.
[4] A.D. Burrows and D.M.P. Mingos, Transition Met. Chem., 1993, 18, 129.
[5] E.G. Mednikov, N.K. Eremenko, Yu. L. Slovokhotov, Yu. T. Struchkov and S.P. Gubin, Koord. Khim., 1987, 13, 979.
[6] R. Vilar, S.E. Lawrence, S. Menzer, D.M.P. Mingos and D.J. Williams, in press.
[7] M.F. Retting and P.M. Maitlis, Inorg. Synth., 1990, 28, 110.
[8] Y. Yamamoto and F. Arima, J. Chem. Soc., Dalton Trans. 1996, 1815.
[9] N.M. Rutherford, M.O. Olmstead and A.L. Balch, Inorg. Chem., 1984, 23, 2833.
[10] F.R. Hartley (editor), Chemistry of the Platinum Group Metals, Recent Developments, Elsevier, Germany (1991).
[11] E. Bririo, A. Ceriotti, R. Della Pergola, L. Garlaschelli, F. Demartin, M. Manassero, M. Sansoni, P. Zanello, F. Laschi and B.T. Heaton, J. Chem. Soc., Dalton Trans., 1992, 3237.
[12] H. C. Clark, A.B. Goel and S. Goel, Inorg. Chem., 1979, 18, 2803.
[13] R. Vilar and D.M.P. Mingos, J. Cluster Sci., 1996, 7, 6663.