| [Related articles/posters: 003 031 042 ] |
Silanols are compounds containing the Si-OH group and are thus analogous to the much more well known alcohols. There are, however, despite their close apparent similarities many differences between the two types of compound. For example, it is easier to prepare silanediols, R2Si(OH)2 and silanetriols, RSi(OH)3 than their carbon analogues, and, unlike alcohols, the silanol group has a strong tendency to undergo self condensation reactions to give compounds containing the siloxane, Si-O-Si, linkage. Such condensation reactions mean that silanol containing species are important industrial intermediates, for example, in the silicone industry polycondensation of silanols leads to the formation of silicones, and the hydrolysis of alkoxysilanes leads to silanols as intermediates in sol-gel processes and in surface functionalisation using silane coupling agents. In addition, the Si-OH group is an important reactive site on the surface of silicate rocks and minerals and is found widely distributed at low concentration in water as Si(OH)4. Recent work has also shown that the breakdown of silicone polymers in the natural environment leads to the formation of low molecular weight silanols such as Me2Si(OH)2 and (HOMe2Si)2O.1 This could have potential environmental implications as silicones are now very widely spread throughout the natural world.
One of the most important aspects of silanol chemistry is the relatively high acidity of the group. Various IR spectroscopic and titration studies have shown that the relative order of acidities for silanols and alcohols is arylsilanols > alkylsilanols > arylcarbinols > alkylcarbinols.2 The high acidity, coupled with the relatively high basicity,2 of the silanol group means that silanols have a strong tendency to form hydrogen bonds both between themselves and to other species containing suitable hydrogen bonding sites. Hydrogen bonding between silicone derived organosilanols, and organic compounds in the environment or silicate surfaces may play an important role in the complete breakdown of silicones to inert inorganic materials.
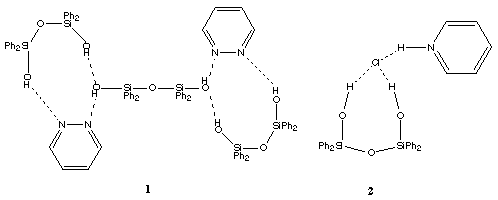
One of the problems in trying to understand the important factors that govern the formation of well defined silanol complexes is that there are many situations where attempts to form a particular complex lead to silanol condensation reactions. For example, in most instances the addition of an amine to methylsilanols such as (HOMe2Si)2O leads to the formation of siloxanes rather than hydrogen bonded complexes as the condensation of silanols is catalysed by both bases and acids. This has led to most of the successful studies being carried out using the more bulky (and hence more stable) aryl silanols, although Selin was able to show that the unsymmetrical disiloxane HOPh2SiOSiMePhOH did form stable complexes with both tributylamine and pyridine.18 Even for arylsilanols condensation reactions may be troublesome for example treatment of (HOPh2Si)2O with either BunNH2 or n-octyl2NH leads to new siloxane bond formation.19
In spite of the problems associated with condensation reactions and with the apparent highly specific structural requirements for the formation of well defined compounds that can readily be isolated we have been able to prepare and structurally characterise a range of novel silanol complexes. The TMEDA complex (Ph3SiOH)2.TMEDA comprises discrete centrosymmetric units in which each Ph3SiOH molecule bonds to a single nitrogen of the TMEDA molecule while, surprisingly, in Ph3SiOH.tris(2-aminoethylamine) which contains four potential hydrogen bonding sites, only one is used, the silanol interacting with a single NH2 group.20 Triphenylsilanol also forms an interesting complex, (Ph3SiOH)2.18-crown-6.(H2O)2, in which the silanol groups hydrogen bonds to the water molecules which, in turn, hydrogen bond to the ether ring.20 The structure of the dioxane complex (HOPh2Si)2O.dioxane is also an infinite chain similar to that in (Ph3SiOH)2.TMEDA.20 We have now extended the range of hydrogen bonded complexes and three new compounds are described below.
The crystalline complex (HOPh2Si)2O.TMEDA is readily formed after addition of hexane to a toluene solution of the silanol and the amine.
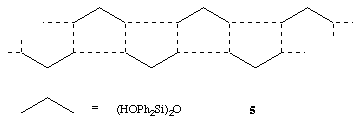
The arrangement is similar to that found for (Ph3SiOH)2.TMEDA except that in the Ph3SiOH complex the presence of only one SiOH group in each silanol molecule means that discrete units are formed rather than infinite chains.20 A linear siloxane linkage has also been found in [(HO)2ButSi]2O which forms hydrogen bonded sheets, 4 with alternate But groups above and below the plane of the sheet.21 The structure of (HOPh2Si)2O itself in the solid state comprises infinite hydrogen bonded chains with the arrangement shown, 5.22 The O...N hydrogen bonded distance in (HOPh2Si)2O.TMEDA is in the range found for similar interactions in the sila-drugs.2
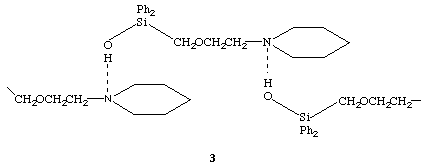
The amine complex [(HOPh2Si)2O]4.[Et2NH]2 can be prepared in a similar way to the TMEDA complex above, the molecular struture and the detail of the hydrogen bonding are shown in the figures.
The X-ray analysis reveals the formation of a 4:2 complex between the siloxanediol and the Et2NH. The asymmetric unit contains four crystallographically independent silanol molecules of similar conformations, each having a pseudo gauche relationship between their hydroxyl groups with respect to the associated Si...Si vectors (torsion angles O-Si...Si-O ranging between 37 and 59 [ring]). The siloxane Si-O-Si angles are in the narrow range 140.5(4) to 145.5(4) [ring] and their Si-O distances range between 1.607(5) and 1.649(5) Å; the Si-OH distances range between 1.586(5) and 1.635(6) Å. The 4:2 complexes in the crystal exist as discrete hydrogen bonded units. Pairs of silanol molecules are linked via OH...O hydrogen bonds (O...O distances ranging from 2.66 to 2.75 Å). These pairs are linked via a combination of OH...N and NH...O hydrogen bonds (OH...N, 2.82 Å, NH...O, 3.14 Å) to one of the Et2NH molecules. The second Et2NH molecule is linked via an OH...N hydrogen bond to just one hydrogen bonded pair of silanol molecules (OH...N, 2.70 Å), the amino hydrogen atom of this second diethylamino molecule being directed back into the 4:2 complex forming a NH...[pi] interaction with one of the phenyl rings on an adjacent silanol molecule (H...[pi], 2.66 Å). Because of the inwardly directing nature of all of the hydrogen bonding interactions, the 4:2 complex presents an essentially hydrophobic exterior and is only involved in weak edge to face interactions between peripheral phenyl rings of adjacent complexes. The discrete units formed by this complex are in contrast to the more usual variety of chain structures formed by the siloxanediols (HOR2Si)2O (R = Me, Et, Prn, Pri, Ph)2 and several of the sila drugs as described above.2
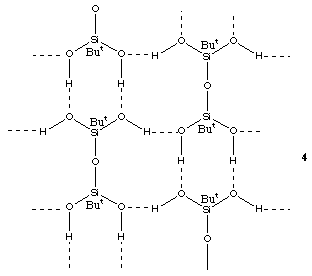
A third amine complex, [(Me3Si)3CSiPh2OH].TMEDA, can be isolated from an ether solution of the amine and the silanol. The X-ray analysis reveals a 2:1 complex, the TMEDA being positioned about a crystallographic inversion centre.
The two silanol units are linked to the TMEDA via OH...N hydrogen bonds (O...N, H...N, 2.75, 1.89 Å, O-H...N, 158 [ring]). There are small departures from tetrahedral geometry at the quaternary carbon centre with angles ranging 106.4(1) to 113.1(1) [ring]. The C-Si distances range between 1.906(3) Å for the SiOH silicon to 1.944(3) Å to one of the SiMe3 groups. The Si-O distance is 1.628(2) Å. Inspection of the packing shows the complexes to exist as essentially discrete units, there being no intemolecular [pi]-[pi] or CH-[pi] interactions. The hydrogen bonding arrangement is similar to that found for (HOPh2Si)2O.TMEDA in that the TMEDA molecules act as bridges between the silanols, but in this case [as for (Ph3SiOH)2.TMEDA20] with only a single SiOH group in each silanol molecule, discrete units rather than infinite chains are formed. (The structure of (Me3Si)3CSiPh2OH itself comprises simple dimers with only a single OH...OH hydrogen bond.23) In contrast to many organosilicon compounds containing the sterically demanding (Me3Si)3C group, it does not, in this case, seem to lead to any unusual structural features.
2. P. D. Lickiss, Adv. Inorg. Chem., 1995, 42, 147.
3. P. I. Prescott and T. G. Selin, US Patent, 3,222,369, 1965; P. I. Prescott and T. G. Selin, US Patent, 3,317,578, 1967.
4. K. A. Ruud, J. S. Sepeda, F. A. Tibbals, and D. C. Hrncir, J. Chem. Soc., Chem. Commun., 1991, 629.
5. M. A. Hossain, M. T. Rahman, G. Rasul, M. B. Hursthouse, and B. Hussain, Acta Crystallogr., Sect C, 1988, C44, 1318.
6. R. Tacke, M. Strecker, W. S. Sheldrick, E. Heeg, B. Berndt, and K. M. Knapstein, Z. Naturf., 1979, 34b, 1279.
7. R. Tacke, M. Strecker, W. S. Sheldrick, L. Ernst, E. Heeg, B. Berndt, C.-M. Knapstein, and R. Niedner, Chem. Ber., 1980, 113, 1962.
8. M. Söderholm, Acta Chem. Scand., 1984, B38, 303.
9. M. Söderholm, Acta Chem. Scand., 1984, B38, 31.
10. M. Söderholm, Acta Chem. Scand., 1984, B38, 95.
11. R. Tacke, H. Lange, W. S. Sheldrick, G. Lambrecht, U. Moser, and E. Mutschler, Z. Naturf., 1983, B38, 738.
12. W. S. Sheldrick, H. Linoh, R. Tacke, G. Lambrecht, U. Moser, and E. Mutschler, J. Chem. Soc., Dalton, 1985, 1743.
13. R. Tacke, J. Pikies, F. Weisenberger, L. Ernst, D. Schomburg, M. Waelbroeck, J. Christophe, G. Lambrecht, J. Gross, and E. Mutschler, J. Organometal. Chem., 1994, 466, 15.
14. P. D. Lickiss and K. M. Stubbs, J. Organometal. Chem., 1991, 421, 171.
15. S. A. Bourne, L. R. Nassimbeni, K. Skobridis, and E. Weber, J. Chem. Soc., Chem Commun., 1991, 282.
16. E. A. Babaian, M. Huff, F. A. Tibbals, and D. C. Hrncir, J. Chem. Soc., Chem. Commun., 1990, 306.
17. S. A. Bourne, L. Johnson, C. Marais, L. R. Nassimbeni, E. Weber, K. Skobridis and F. Toda, J. Chem. Soc., Perkin Trans. 2, 1991, 1707.
18. T. G. Selin, US Patent, 3,231,575, 1966.
19. L. D. Cother and P. D. Lickiss, unpublished results.
20. N. Choi, L. D. Cother, P. D. Lickiss and M. McPartlin, manuscript in preparation.
21. P. D. Lickiss, S. A. Litster, A. D. Redhouse and C. A. Wisener, J. Chem. Soc., Chem. Commun., 1991, 173.
22. M. A. Hossain and M. B. Hursthouse, J. Crystallogr. Spectrosc. Res., 1988, 18, 227; V. E. Shklover, Yu. T. Struchkov, I. V. Karpova, V. A. Odinets and A. A. Zhdanov, J. Struct. Khim, (Engl. Transl.), 1985, 26, 251.
23. A. I. Al-Mansour, S. S. Al-Juaid, C. Eaborn and P. B. Hitchcock, J. Organometal. Chem., 1994, 480, 139.
24. F. J. Feher and T. A. Budzichowski, Polyhedron, 1995, 14, 3239.