| [Related articles/posters: 077 014 007 ] |
Jordis, Ulrich*); Rizzi, Andreas**); Grohmann, Franz*);
*) Institute of Organic Chemistry, Vienna University of Technology,
Getreidemarkt 9, A 1060 Vienna, Austria;
**) Institute of Analytical Chemistry, University Vienna, Währingerstr. 8, A 1090 Vienna;
Polymerization of methacrylic acid and trimethylolpropanetrimethacrylate in the presence of (-)-galanthamine as imprinting template resulted in a polymer that, after extractive removal of the template, was used for batch- and preliminary HPLC-separation of (+-)-Galanthamine.
Introduction
The concept of imprinting of synthetic polymers using molecular templates has been reviewed [1] and patents for applications have been published [2].
The aim of our work was to investigate the possibility of preparing custom-designed polymers for the chiral separation of a target compound. While many chiral solid phases exist already for the separation of enantiomers by chiral HPLC, there remains enough trial and error in finding a suitable chiral stationary phase, and typically for new separations a number of (expensive) phases must be evaluated. We wanted to circumvent this problem by rationally designing solid phases for the resolution for a defined separation problem. Such an approach would be justified, if the separation problem was to be repeated on a routine basis, as might be the case e.g. for the separation of biologically active compounds or for the routine batch analysis in a production scenario. In addition it is conceivable to use such imprinted polymers in a "polishing step" for the removal of undesired enantiomeric impurities.
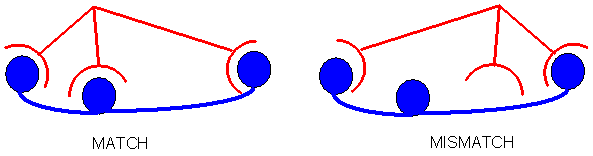
The recognition of the template within the polymer matrix can be achieved via non-covalent bonds comprising electrostatic-, hydrophobic-, charge-transfer- (e.g. hydrogen bonds) or coordinated metal-bonds. For a chiral recognition a minimum of three binding sites for each molecule must exist:
Fig. 1: Chiral recognition
| (-)-Galanthamine | (+)-Galanthamine |
The best result obtained so far were enantioselectivity coefficients defined as mass ratios of polymer-bound anantiomers to non-bound enantiomers
[(m(-)pol / m(-)sol)/m(+)pol/m(+)sol)] between 1.3 and 2.0 depending on the preparation procedure of the polymers. With the phase ratio chosen such a selectivity corresponds to a 2:1 enrichment of the non-imprinted enantiomer in the supernatant as analyzed by chiral capillary electrophoresis (CE). A typical electropherogram is shown in fig.2. Fig. 2: Chiral CE of (-)- and (+)-galanthamine [4]Experimental
Molecular imprinted polyacrylate
A solution of (-)-galanthamine (129 mg, 0.45 mmol), methyl acrylate (370 mg, 4.4 mmol), trimethylolpropane trimethacrylate (4.6g, 13.6 mmol) 2,2'-azobis(isobuyronitrile) (30 mg) in chloroform (6 ml) was degassed for 15 min using argon. The solution was polymerized for 18 hrs. at 0-20° using a UV lamp resulting in a yellowish, clear, brittle powder. The polymer was filtered, washed, dried and ground, first manually using a porcelane mortar and piston, than in a mechanically driven agate mortar for15 min at 100 rpm. The powder was wet-sieved by brushing a suspension of the polymer in 800 ml EtOH/water 1:1) over a 45mm sieve. The residue from this sieving procedure was used for batch-binding experiments (see below).
Batch-binding of rac.-Galanthamine
For these experiments the >45mm particles were used and any remainig template was removed by Soxleth-extraction (24 hrs., 15% AcOH in chloroform). The molecular imprinted polymer was dried in vacuo. 30 mg of this material was sonicated after addition of 1 ml of rac.-galanthamine (1.6 mmol/ml) for 5, 10, 30, 60 and 180 min and the supernatant analyzed by chiral CE for the (-)- and (+)-galanthamine ratio.
First attemps for the HPLC resolution of rac.-galanthamine using a molecular imprinted polymer
To prepare material suited for HPLC-columns, the > 45mm from residue was dried under an IR-lamp and the grinding and sieving procedure was repeated three times. The combinded fines thus obtained using the 45mm sieve were wet-sieved using a 25mm sieve. The 25mm-45mm particles were further purified by sedimentation of a sonicated suspension in acetonitrile using a glass column (20 x 560 mm) [3]. The material thus obtained contained very few particles in the 3mm range as viewed by microscope. 980 mg of this material were used to fill a 4mm HPLC column and any remaining template was removed using MeOH/AcOH 9:1 (250 ml).