New Pyrimidine Cyclonucleosides with Hydrogenated Aglycones: Synthesis and Structure
aDepartment of Organic Chemistry, State Academy of Fine Chemical
Technology, Vernadsky Avenue 86, Moscow 117571, Russian Federation;
bInstitute of Molecular Biology RAS, Vavilova Street 32, Moscow
117984, Russian Federation
 Introduction
Introduction
 Results
and Discussion
Results
and Discussion
 Synthesis
of Ribosides
Synthesis
of Ribosides
 Synthesis
of Xylosides
Synthesis
of Xylosides
 Conclusion
Conclusion
 References
References
 Introduction
Introduction
Base-modified nucleoside analogues received significant interest as potential
biologically active substances. However, only few investigations were concerned
with the syntheses of nucleoside analogues containing hydrogenated aglycones.
Some of these compounds possess antiviral and antitumor activities, enzymatic
inhibitory properties, etc. In continuation of our studies on hydrogenated
heterocycles with two heteroatoms at the 1,3 positions [1]
we interested in synthesis of nucleoside analogues containing these heterocycles
as aglycones. In result of our interest we developed two methods for the
synthesis of N-glycosides of 4-hydroxyhexahydropyrimidine-2-thiones [2,
3] as outlined in Scheme 1.
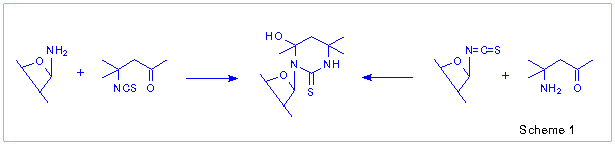
The first method of the nucleoside synthesis is based on the reaction
of readily available glycosylamines with b-isothiocyanatoaldehydes
or b-isothiocyanatoketones [2].
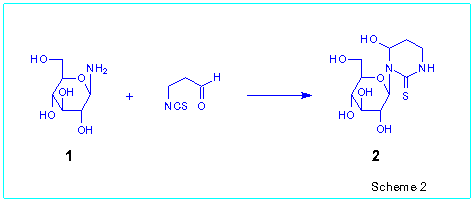
For example, b-D-glucopyranosylamine 1
easily reacted with 3-isothiocyanatopropanal in pyridine to give 3-(b-D-glucopyranosyl)-4-hydroxyhexahydropyrimidine-2-thione
2 in 79 % yield (Scheme 2).
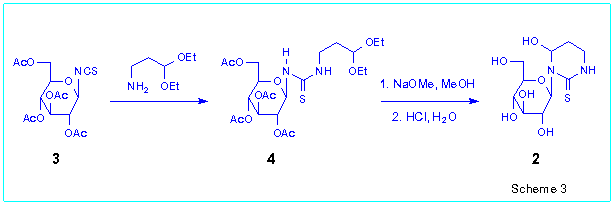
The second method includes the reaction of peracetylglycosylisothiocyanates
with b-aminoaldehydes or b-aminoketones
as well as their derivatives [3]. The synthesis
of N-glucoside 2 starting from readily available 2,3,4,6-tetra-O-acetyl-b-D-glucopyranosylisothiocyanate
3 can serve as an illustration of this approach (Scheme 3). Thus
the reaction of 3 with 3,3-diethoxypropane-1-amine gave the glucosylthiourea
4 which after deprotection was transformed into the target nucleoside
2 in 63 % overall yield.
The described methods were also applied to the syntheses of 3-(b-D-galactopyranosyl)-4-hydroxy-
and 4-hydroxy-3-(D-ribosyl)hexahydropyrimidine-2-thiones [2,
3].
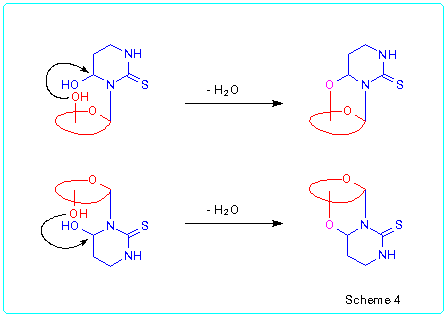
One of the structural peculiarities of the obtained nucleoside analogues
lies in the presence of semiacetal hydroxyl group at the pyrimidine ring
as well as several hydroxyl groups in sugar moiety. Earlier we demonstrated
that 4-hydroxyhexahydropyrimidine-2-thiones easily react with a large variety
of nucleofiles (alcohols, amines, etc.) to give the products of substitution
of the hydroxyl group [4]. We proposed that intramolecular
nucleophilic substitution of the hydroxyl group of pyrimidine moiety could
take place in the case of some 3-glycosyl-4-hydroxyhexahydropyrimidine-2-thiones
to produce the corresponding cyclonucleosides (Scheme 4).
Here we report the synthesis of some new types of pyrimidine cyclonucleosides
with hydrogenated aglycones starting from partly protected ribo-
and xylofuranosylamines.
 Results and Discussion
Results and Discussion
 Synthesis of Ribosides
Synthesis of Ribosides
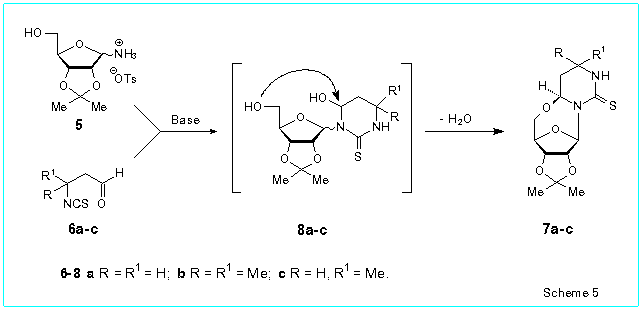
We found that 2,3-O-isopropylidene-D-ribofuranosylammonium p-toluenesulfonate
5 reacts readily with the b-isothiocyanatoaldehydes
6a-c in the presence of bases (triethylamine or pyridine) to produce
4,5'-anhydro-4-hydroxy-3-(2,3-O-isopropylidene-b-D-ribofuranosyl)hexahydropyrimidine-2-thiones
7a-c in 66-79 % isolated yields (Scheme 5). Evidently, the 4,5'-anhydroribosides
7a-c are formed in result of spontaneous intramolecular nucleophilic
replacement of the hydroxyl group at the pyrimidine ring in b-anomers
of the intermediate 4-hydroxy-3-(D-ribofuranosyl)hexahydropyrimidine-2-thiones
8a-c.
It should be noted that the reaction of 5 with 6a,b proceeds
with complete diastereoselectivity to give (S)-configuration of the chiral
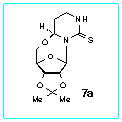
center C(4) of the nucleosides 7a,b. The structures of these compounds
were assigned on the bases of their NMR spectra as well as NOE measurements
on the nucleoside 7a. In addition the structure of 7a was
unambiguously established by a single crystal X-ray
diffraction study.
The reaction of ribosylamine 5 with 6c also gives exclusively
(4S)-4,5'-anhydroriboside 7c. However, as we used racemic 3-isothiocyanatobutanal
6c, the nucleoside 7c are obtained as a mixture of
two diastereomers (1:1) which differ by configuration at the C(6). These
diastereomers were separated by column chromatography.
The next step of our investigation included removal of the isopropylidene
protective group from 7a-c. However, to our surprise, heating of
7a in 25 % aqueous AcOH at 95 oC during 5 h failed to give the expected
(4S)-4,5'-anhydro-4-hydroxy-3-(b-D-ribofuranosyl)hexahydropyrimidine-2-thione
10a. Instead of 10a, 4,2'-anhydro-4-hydroxy-3-(a-ribofuranosyl)hexahydropyrimidine-2-thione
9a was obtained as the main product (57 % isolated yield) of the
reaction (Scheme 6). Besides 9a, the products of cleavage of the
glycosidic bond namely 4-hydroxyhexahydropyrimidine-2-thione (12 %) and
D-ribose were also isolated from the reaction mixture.
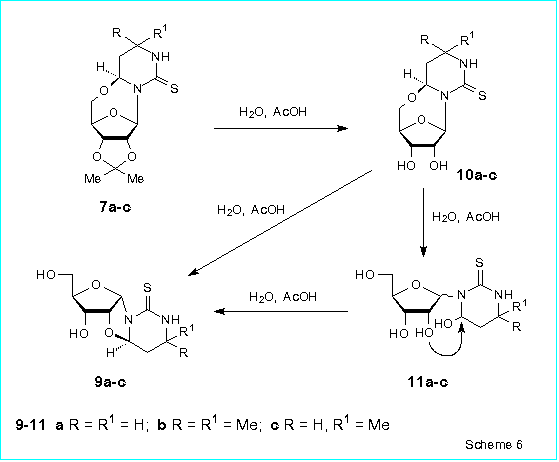
We found that this unusual conversion of the 4,5'-anhydroriboside 7a
into the 4,2'-anhydroriboside 9a involves initial deprotection of
the compound 7a to yield the b-riboside
10a. Subsequent cleavage of the oxygen-bridge of the nucleoside
10a leads to 4-hydroxy-3-(D-ribosyl)hexahydropyrimidine-2-thione
11a which is an equilibrium mixture of a-
and b-ribopyranosides and a-
and b-ribofuranosides [3].
At last the a-ribofuranose form of 11a
gives the final product 9a in result of intramolekular cyclization
(Scheme 6).
Use of shorter reaction time (less than 4.5-5 h) showed that the reaction
mixtures consist of the starting material 7a, the final product
9a as well as two other nucleosides namely the 4,5'-anhydro-b-riboside
10a and the riboside 11a. All the components of the
reaction mixtures (7a, 9a, 10a, 11a, D-ribose
and 4-hydroxyhexahydropyrimidine-2-thione) were isolated by column chromatography.
Ratio of all these components depends on reaction time. For example, isolated
yields of 7a, 9a, 10a, 11a and 4-hydroxyhexahydropyrimidine-2-thione
were 25, 43, 16, 4 and 3 % (reaction time 1.17 h) or 20, 47, 13, 8 and
6 % (reaction time 1.58 h). Ratios of 7a, 9a and 10a
determined by NMR spectroscopy were 31:46:23 (reaction time 1.17 h), 17:65:18
(reaction time 1.58 h), 7:85:8 (reaction time 2.75 h) or ~0:100:~0 (reaction
time 5 h).
The proposed route of transformation of 7a into 9a was
also confirmed by preparation of the 4,2'-anhydroriboside 9a starting
from the ribosides 10a or 11a (25 % AcOH, 95 oC).
The similar reactions proceed by heating of the 4,5'-anhydroribosides
7b,c in 25 % aqueous acetic acid to afford the 4,2'-anhydroribosides
9b,c as the final products. However, stability of the oxygen-bridge
to the hydrolytic cleavage is increased in the sequence of the compounds
10a>10c>10b. Actually, the heating of 7c for
1.17 h in 25 % AcOH gave 29, 18, 33, 14 and 5 % isolated yields for 7c,
9c, 10c, 11c and trans-4-hydroxy-6-methylhexahydropyrimidine-2-thione
correspondingly. Transformation of 7b into 9b proceeds in
25 % AcOH at 95 oC very slowly. For example, after 4.5 h we were able to
isolate the 4,2'-anhydroriboside 9b only in 14 % yield whereas 10b
in 54 % yield.
It is essential to note that in all the studied cases the 4,2'-anhydroribosides
9a-c are formed exclusively as one of two possible diastereomers.
We showed that the obtained ribosides 9a-c have (R)-configuration
at the C(4). The structures of these compounds were assigned on the bases
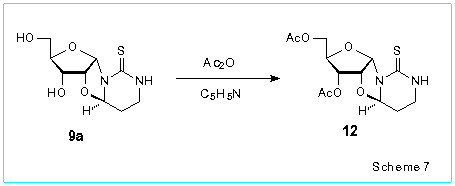
of their NMR spectra as well as NOE measurements on the nucleoside 12
synthesized in 88 % yield by treatment of 9a with acetic anhydride
in pyridine.
 Synthesis of Xylosides
Synthesis of Xylosides
We showed that 3,5-O-isopropylidenexylofuranosylammonium p-toluenesulfonate
13 reacts readily with the b-isothiocyanatoaldehydes
6a-c in the presence of triethylamine in chloroform to afford 4,2'-anhydro-4-hydroxy-3-(3,5-O-isopropylidene-a-D-xylofuranosyl)hexahydropyrimidine-2-thiones
14a-c in 43-59 % isolated yields (Scheme 8). These compounds are
formed in result of spontaneous intramolecular nucleophilic replacement
of the hydroxyl group at the pyrimidine ring in a-anomers
of the intermediate 4-hydroxy-3-(D-xylofuranosyl)hexahydropyrimidine-2-thiones
15a-c. The reactions of 13 with 6a-c proceed with
complete diastereoselectivity to give (R)-configuration of the chiral center
C(4) of the nucleosides 14a-c.
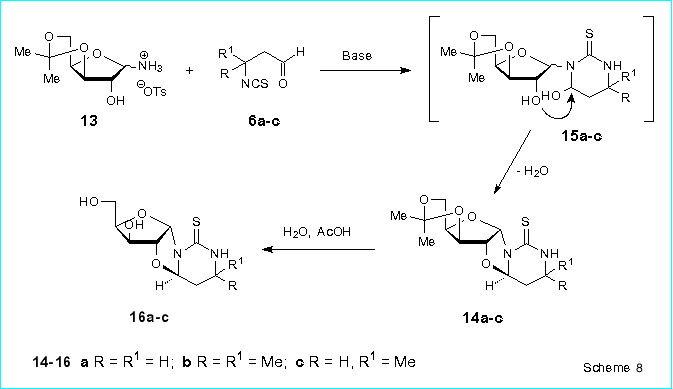
Deprotection of the 4,2'-anhydroxylosides 14a-c proceeds very
readily by heating of these compounds in 25 % aqueous acetic acid (95 oC,
5 min) or in ethanol in the presence of HCl (reflux, 5 min) to produce
(4R)-4,2'-anhydro-4-hydroxy-3-(a-D-xylofuranosyl)hexahydropyrimidine-2-thiones
16a-c in 82-100 % isolated yields.
 Conclusion
Conclusion
Thus reaction of partly protected ribo- and xylofuranosylamines with
b-isothiocyanatoaldehydes gives convenient stereoselective
access to new types of cyclonucleosides containing hydrogenated pyrimidine
aglycone. Synthesized cyclonucleosides are of great interests as starting
compounds for syntheses of new nucleoside analogues as well as potential
biologically active substances.
 References
References
1. Shutalev, A.D.; Kishko, E.A.; Sivova,
N.V.; Kuznetsov, A.Yu. Molecules 1998, 3, 100-106;
Shutalev, A.D.; Kuksa, V.A. Khim. Geterotsikl. Soedin. 1997,
105-109; Shutalev, A.D.; Sivova, N.V. Khim. Geterotsikl. Soedin.
1996, 1337-1342; Shutalev, A.D.; Kuksa, V.A. Khim. Geterotsikl.
Soedin. 1995, 97-103; Shutalev, A.D.; Pagaev, M.T.; Ignatova,
L.A. Khim. Geterotsikl. Soedin. 1994, 1093-1104;
Shutalev, A.D. Chemistry of Heterocyclic Compounds, 1993,
29, 1192-1199. Engl. transl. from Khim. Geterotsikl. Soedin.
1993, 1389-1397; Shutalev, A.D. Chemistry of Heterocyclic Compounds,
1993, 29, 1421-1425. Engl. transl. from Khim. Geterotsikl.
Soedin. 1993, 1645-1649; Shutalev, A.D.; Pagaev, M.T.; Ignatova,
L.A. Zh. Org. Khim. 1991, 27, 1274-1285.
2. Shutalev, A.D.; Ignatova, L.A.; Unkovsky, B.V. USSR 1392864
(Cl C 07 H 19/06), 15 Sept 1987;
Shutalev, A.D. unpublished data.
3. Ignatova, L.A.; Shutalev, A.D.; Unkovsky, B.V.; Sinilova,
N.G.; Duplischeva, A.P. Khimiko- farmatsevtich. zhurnal, 1985,
1447-1453; Shutalev, A.D.; Ignatova, L.A.; Unkovsky, B.V. Khim. geterotsicl.
soedin. 1984, 548-551; Shutalev, A.D.; Ignatova, L.A.; Unkovsky,
B.V. Khim. geterotsicl. soedin. 1982, 825-829; Shutalev,
A.D.; Ignatova, L.A.; Unkovsky, B.V. Khim. geterotsicl. soedin. 1982,
269.
4. Shutalev, A.D.; Alekseeva, S.G. Khim. Geterotsikl. Soedin.
1995, 377-380; Shutalev, A.D.; Komarova, E.N.; Ignatova, L.A.
Chemistry of Heterocyclic Compounds 1993, 29, 1182-1191.
Engl. transl. from Khim. Geterotsikl. Soedin. 1993, 1378-1388;
Shutalev, A.D.; Komarova, E.N.; Pagaev, M.T.; Ignatova, L.A. Chemistry
of Heterocyclic Compounds 1993, 29, 1077-1086. Engl.
transl. from Khim. Geterotsikl. Soedin. 1993, 1259-1270;
Shutalev, A.D.; Ignatova, L.A. Khim. Geterotsikl. Soedin. 1991,
228-236; Ignatova, L.A.; Shutalev, A.D.; Shingareeva, A.G.; Dymova, S.F.;
Unkovsky, B.V. Khim. Geterotsikl. Soedin. 1985, 260-266.

![]() Introduction
Introduction
![]() Results
and Discussion
Results
and Discussion
![]() Synthesis
of Ribosides
Synthesis
of Ribosides
![]() Synthesis
of Xylosides
Synthesis
of Xylosides
![]() Conclusion
Conclusion
![]() References
References









