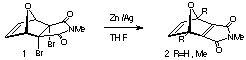The Control of Stereoselectivities in the Diels-Alder Reactions of N-Substituted Pyrroles and N-Substituted Isoindoles
Guangxing Sun, Douglas N. Butler, Ronald N. Warrener, Davor Margetic, John R. Malpass
Centre for Molecular Architecture, Central Queensland
University, Rockhampton, Queensland 4702, Australia
Abstract: Pyrrole and its N-substituted derivatives
are not recognised as good Diels-Alder dienes and only a small
number of cycloadditions involving pyrroles with dienophiles have
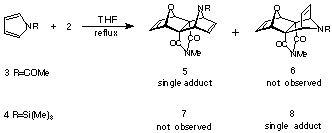
been reported1-3. In this paper, we report new Diels-Alder
reactions of N-acetyl and N-trimethylsilyl pyrroles
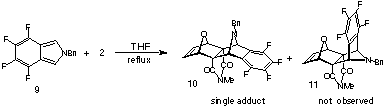
and N-benzyl-tetra- fluoroisoindole with 7-oxanorbornadienomaleimide
ONM 2(R=H) where the addition stereoselectivity is affected
by the N-substituents of the pyrrole. Semiempirical (PM3)
calculations in support of these findings are also presented along
with preliminary results for the additions of the above pyrroles
to the related dienophile ONM 2(R=Me).
Keywords: Pyrroles; Isoindoles; Diels-Alder Reactions;
Stereoselectivities
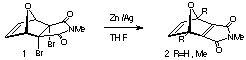
References:
1. Wirth D.; Lemal D.M. J. Am. Chem. Soc. 1982,
104, 847-848
2. Pitt, I. G.; Russell, R. A.; Warrener R. N. Synth. Commun.
1986, 16, 1627-1634
3. Chen, Z.; Trudell M. L. Chem. Rev. 1996, 96,
1179-1193
4. Warrener, R. N. Chemistry in Australia 1992,
59, 578-581
Main Article