Influence of Microwave Irradiation on the Rate of Coumarin Synthesis by the Knoevenagel Condensation.
Institute of Organic Chemistry, Politechnika Krakowska ul. Warszawska 24, 31-155 Krakow, Poland;
e-mail: pcbogdal@cyf-kr.edu.pl
Abstract: The influence of microwave irradiation on the kinetic of the
condensation
reaction of salicylaldehyde and ethyl malonate was investigated. The rate
constants for the reaction at various temperatures were measured in experiments
conducted in a water bath and the Arrhenius parameters were calculated. The
same reactions were carried out under microwave irradiation with different
temperature profiles and the final product, ethyl 3-ethoxycarbonylcoumarin,
concentrations were determined. The measured coumarin concentration were
higher than those calculated by computer modeling of the reaction using the
Arrhenius parameters obtained from the water bath experiments.
The relationship between the rates of chemical reactions and temperature is defined by
the Arrhenius equation. Therefore determining whether the reaction rate observed under
microwave irradiation is the same as that expected from the Arrhenius equation should
resolve the debate on the observed rate enhancements.
This report examines the influence of microwave radiation on the kinetics of the condensation
of salicylaldehyde with ethyl malonate in toluene. Ethyl 3-ethoxycarbonylcoumarin
was the final product of the condensation (Fig.1).
The reaction was selected because it proceeded relatively slowly under reflux
in the presence of piperidine as a catalyst.

|
| Fig.1. |
The condensation reaction of salicylaldehyde and ethyl malonate
under microwave irradiation.
|
This reaction was first investigated under conventional conditions to establish the
Arrhenius parameters. Solutions containing salicylaldehyde, ethyl malonate and piperidine
were prepared and then placed in an open vessel in a thermostated water bath.
Samples were withdrawn at intervals and were analyzed by GC/MS. The overall rate constant,
kobs, was obtained by least-squares fitting method.
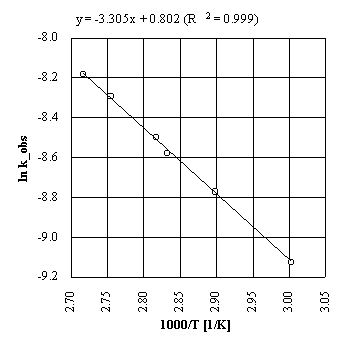
The Arrhenius parameters for this reaction were determined from a plot of ln kobs
versus 1/T. The Arrhenius plot is presented in Figure 2.
|
|
Fig.2. |
Arrhenius plot for the condensation reaction of salicylaldehyde with
ethyl malonate under microwave irradiation.
|
A kinetic analysis of this reaction was then made in the monomode microwave reactor
Synthwave 402 (Prolabo). Solutions of salicylaldehyde, ethyl malonate and piperidine
were prepared and then placed in an open vessel adapted to the reactor. The recorded
temperature time profiles and the Arrhenius parameters were then used to predict the
final ethyl 3-ethoxycarbonylcoumarin concentrations of the reaction. The reaction mixtures
were analyzed by GC/MS and the analytically determined final ethyl 3-ethoxycarbonylcoumarin
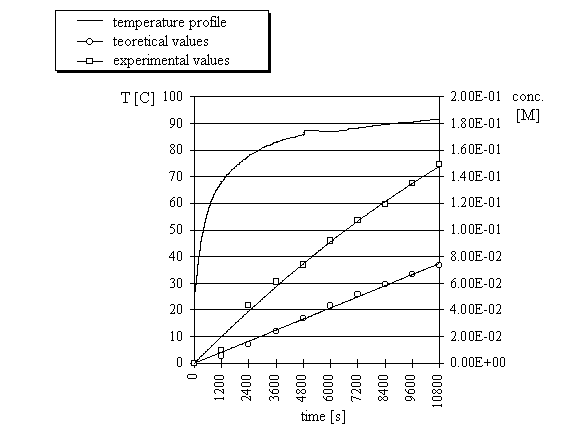
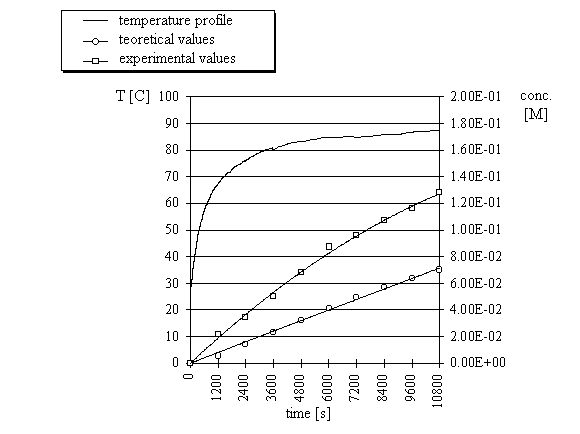
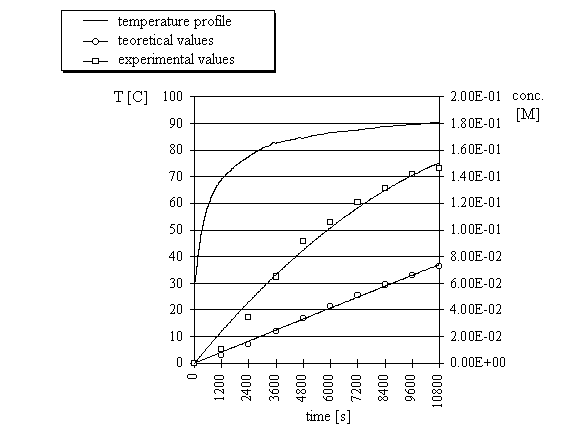
concentrations were compared to the calculated concentrations. Three reactions were run with
three different temperature profiles (Fig. 3-5) and in each case the concentrations obtained
from microwave experiments were significantly higher than the computed concentrations.
|
| Fig.3. |
Computed and experimental concentrations of ethyl 3-ethoxycarbonylcoumarin
and temperature profile of the condensation
reaction of salicylaldehyde with ethyl malonate under microwave irradiation (experiment 1).
|
|
| Fig.4. |
Computed and experimental concentrations of ethyl 3-ethoxycarbonylcoumarin
and temperature profile of the condensation
reaction of salicylaldehyde with ethyl malonate under microwave irradiation (experiment 2).
|
|
| Fig.5. |
Computed and experimental concentrations of ethyl 3-ethoxycarbonylcoumarin
and temperature profile of the condensation
reaction of salicylaldehyde with ethyl malonate under microwave irradiation (experiment 3).
|
In conclusion, it is demonstrated that microwave radiation acts to enhance the kinetic
of Knoevenagel condensation over that obtained for conventional
treatments by 2 to 3 times, depending on the measured temperature of
the reaction. The results presented here, combined with previous data for a range
of other systems, suggests that this acceleration may be a general
phenomenon for systems where dipole moment react but the magnitude of the
acceleration will vary.




