| [Related articles/posters: 113 088 103 ] |
Abstract:
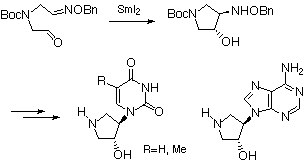
SmI2-induced 5-exo-trig radical cyclization of oxime ether intramolecularly connected with the formyl group was found to be particularly effective for preparing cyclic trans-amino alcohol in good chemical yield with a high degree of stereoselectivity. The method was successfully applied to the synthesis of 4-pyrimidinyl- and 4-purinylpyrrolidin-3-ol nucleoside analogues.
(1) Introduction
(2) SmI2-induced cyclization of oxime ether connected with formyl group
(3) Synthesis of 4-pyrimidinyl- and 4-purinylpyrrolidin-3-ol nucleoside analogues
(4) Conclusion
(5) Experimental section
(6) Acknowledgements
(7) References and notes
(1) Introduction
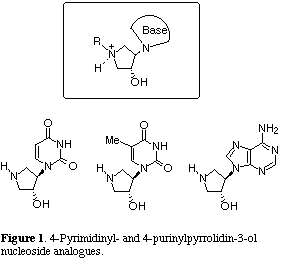
Modifications of nucleoside framework in which the furanose oxygen atom was replaced by another hetero atom have recently led to the discovery of a novel class of potential anticancer and antiviral agents.[1] It is proposed that a slight change in the size and electronegativity of the hetero atom would lead to the compounds that would increase biological activity and metabolic stability and decrease the toxicity of 4'-oxo analogues. Since incorporation of a nitrogen atom into furanose ring has not been extensively investigated,[2] we have started synthetic studies of a new class of 4-pyrimidinyl- and 4-purinylpyrrolidin-3-ol nucleoside analogues which may have wide-ranging therapeutic potential based on the following reasons (Figure 1). Replacement of the 4'-chiral carbon atom of a nucleoside by a nitrogen atom would provide the system with more conformational flexibility. Moreover, positive charge associated with protonation to the nitrogen atom on pyrrolidine ring would be expected to play an important role on the inhibition of viral or cellular enzymes which are essential for viral replication.[3] Additionally, an amino functionality in these designed analogues can also allow ready access to introduction and modification of the substituent R on the nitrogen atom, thus leading to the formation of a variety of nucleoside analogues. We now describe the synthesis of a novel class of 4-pyrimidinyl- and 4-purinylpyrrolidin-3-ol nucleoside analogues via the SmI2-induced radical cyclization of oxime ether intramolecularly connected with the formyl group.
(2) SmI2-induced cyclization of oxime ether connected with formyl group
Strategies involving radical reactions have become preeminent tools in organic synthesis.[4] Free radical-mediated cyclization has particularly developed as a powerful method for preparing various type of cyclic compounds via carbon-carbon bond-forming processes. Although a number of extensive investigations into radical reaction were reported in recent years, the majority of these employ methods utilizing conventional radical acceptors such as alkenes or typical radical precursors such as halides, selenides and xanthates.[5] It is important to note that one drawback in the traditional procedures using such radical acceptors and precursors is loss of the inherent functional groups. Our laboratory is interested in developing effective and convenient methods for the synthesis of highly functionalized cyclic compounds. For this purpose, we have focused our efforts on the radical reactions using aldehydes and ketones as a radical precursor and/or oxime ether group as a radical acceptor.[6, 7] We recently reported the first example of the tributyltin hydride-induced radical cyclization of oxime ethers which were intramolecularly connected with aldehyde or ketone.[8] This radical cyclization would be initiated by the addition of stannyl radical, generated from Bu3SnH and AIBN, to the oxygen atom of the carbonyl group to form the O-stannyl ketyl radical which would be then intramolecularly trapped by the oxime ether group. Our newly-developed reaction is a facile and practical method to prepare the highly functionalized 5-, 6- and 7-membered compounds that retain the hydroxyl and amino functional groups, although a mixture of two diastereoisomeric products was obtained with low stereoselectivity. We describe here the stereoselective radical cyclization of oxime ether intramolecularly connected with the formyl group based on the chemistry of samarium (II) diiodide (SmI2).

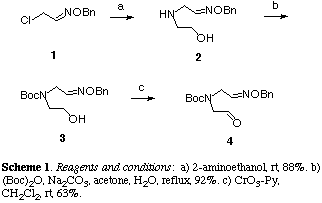
Preparation of oxime ether (4), which is a requisite substrate for the following radical cyclization, is shown in Scheme 1. Treatment of chloroacetaldoxime ether (1)[9] with commercially available 2-aminoethanol gave the secondary amine (2) in 88% yield as an E/Z mixture in the geometry of the oxime ether group in 3:2 ratio.[10] The amine (2) was protected with di-tert-butyl dicarbonate under the Schotten-Baumann type conditions to afford the N-Boc derivative (3) in 92% yield. Mild oxidation of (3) with chromium trioxide-pyridine provided the unstable aldehyde (4) in 63% yield in favor of the E isomer in 5:4 ratio.
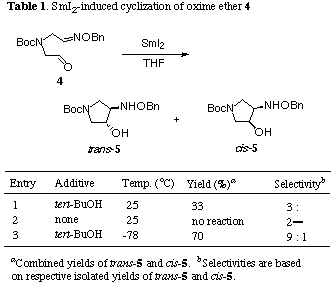
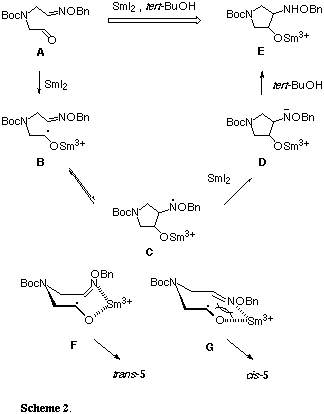
In order to improve the stereoselectivity in radical cyclization of (4) using stannyl radical,[11] we investigated the intramolecular reductive coupling between the formyl and oxime ether groups in the aldehyde (4) based on the chemistry of SmI2 (Table 1). This unique one-electron reducing agent has been recently used for the radical reaction with a high degree of chemo-, regio- and stereoselectivities.[12] Treatment of an E/Z mixture of (4) with commercially available 0.1 M solution of SmI2 in THF (3.0 equiv.) at 25 oC underwent smooth cyclization to give a 3:2 mixture of two cyclized products trans-(5) and cis-(5) in 33% combined yield in favor of trans-amino alcohol (entry 1).[13] No reaction was observed in the absence of tert-BuOH, thus a proton donor such as tert-BuOH was found to be essential for SmI2-induced ring formation (entry 2). Each isomer could be easily separated by flash column chromatography and relative stereostructures were firmly established by the formation of the corresponding acetonide in cis-(5) while trans-(5) recovered completely under the same reaction conditions. The stereoselectivity and chemical yield were shown to be dependent on the reaction temperature, thus changing the temperature from 25 to -78 oC led to an effective increase in trans/cis selectivity to 9:1 and combined yield to 70%, better than that obtained by stannyl radical-promoted reaction (entry 3).[11] Interestingly, this 5-exo-trig radical cyclization took place smoothly even in the absence of HMPA, in contrast to the analogous 7-exo-trig radical cyclization in our previous experiment.[14] A similar result has been recently reported by Chiara in reductive coupling of carbonyl-tethered oxime ethers for the synthesis of aminocyclopentitols.[15] The rationale of the reaction pathway of this reductive coupling reaction is that single-electron transfered to the formyl group to form a ketyl radical (B) which then attacked the oxime ether group (Scheme 2). In this reaction, the oxime ether group acts as an excellent radical acceptors because of the extra stabilization of the intermediate aminyl radical (C) provided by the lone pair on the adjacent oxygen atom. The process was completed by SmI2-induced reduction of the intermediate aminyl radical (C) followed by protonation of the resulting amide anion (D) by tert-BuOH. Therefore, a twofold excess of SmI2 is theoretically necessary for the successful cyclization. The preferential formation of trans-(5) could be explained by assuming both the electronic and steric repulsions between the ketyl radical moiety and the oxime ether group, as indicated in the transition states of the chairlike conformations (F) and (G), of which the former (F) would be more stable than the latter (G).
(3) Synthesis of 4-pyrimidinyl- and 4-purinylpyrrolidin-3-ol nucleoside analogues
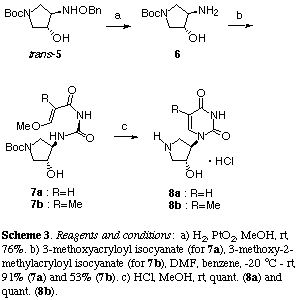
The synthesis of uridine, thymidine and adenosine analogues was achieved by the standard pyrimidine and purine rings construction method. Hydrogenolysis of the benzyloxyamino group in trans-(5) in the presence of platinum dioxide afforded the amino alcohol (6) in 76% yield which was then treated with the corresponding isocyanates[16] in benzene-DMF at low temperature to give the acryloylureas (7a) and (7b) in 91 and 53% yields, respectively (Scheme 3). Acid-catalyzed cyclization of (7ab) gave the uridine and thymidine analogues (8ab) in high yields as a result of concomitant deprotection of the N-tert-butoxycarbonyl group.
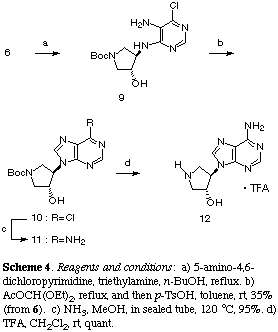
Refluxing a solution of a mixture of (6), commercially available 5-amino-4,6-dichloropyrimidine and triethylamine in n-BuOH produced a common precursor (9) which was then subject to the reaction with diethoxymethyl acetate followed by treatment with p-TsOH in toluene to afford purine derivative (10) in 35% yield from (6) (Scheme 4). Treatment of (10) with methanolic ammonia in sealed tube at 120 oC gave a 95% yield of (11) which was then deprotected by treatment with trifluoroacetic acid to afford the desired adenosine analogue (12) in almost quantitative yield. Evaluation of the biological activities of three products (8a), (8b) and (12) thus prepared is now in progress.
(4) Conclusion
We established 5-exo-trig radical cyclization of oxime ether connected with the formyl group by use of samarium diiodide, providing a powerful method for preparing 5-membered cyclic compounds bearing the hydroxyl and amino functional groups in good chemical yield with a high degree of stereoselectivity. The method was successfully applied to the synthesis of 4-pyrimidinyl- and 4-purinylpyrrolidin-3-ol nucleoside analogues.
(5) Experimental section
General. 1H NMR spectra were measured using Varian Gemini-200 (200 MHz), Varian Gemini-300 (300 MHz) and VXR-500 (500 MHz) instruments. 13C NMR spectra were measured using Varian Gemini-200 (50 MHz) and VXR-500 (125 MHz). IR spectra were measured with a Perkin Elmer 1600 FTIR machine and mass spectra were taken by Hitachi M-4100 spectrometer. Mps were determined with a Kofler-type hot-stage apparatus and are uncorrected. For flash column chromatography, E. Merck Kieselgel 60 (230-400 mesh) was used.
N-(2-Hydroxyethyl)aminoethanal O-Benzyloxime (2). To 2-aminoethanol (10 ml, 166 mmol) was added chloroacetaldehyde O-benzyloxime 1[9] (10 g, 54.5 mmol) under a nitrogen atmosphere at 0 deg.C. After stirring at rt for 12 h, the reaction mixture was added to saturated aqueous NaHCO3 and CH2Cl2. The layers were separated and the aqueous phase was extracted with CH2Cl2. The combined organic phase was washed with brine, dried over Na2SO4 and concentrated at reduced pressure. Purification of the residue by flash column chromatography (CHCl3/MeOH 30:1 to CHCl3/MeOH 15:1) afforded 2 (10 g, 88%) as colorless crystals and a 3:2 mixture of E/Z-oxime. mp 58.5-60 oC (AcOEt/hexane). IR (CHCl3) 3600-3300 (OH, NH) cm-1. 1H NMR (CDCl3) delta; 7.49 (3/5H, t, J=5.2 Hz), 7.38-7.25 (5H, m), 6.79 (2/5H, t, J=4.4 Hz), 5.10 (4/5H, s), 5.07 (6/5H, s), 3.65-3.57 (2H, m), 3.56 (4/5H, d, J=4.4 Hz), 3.38 (6/5H, d, J=5.2 Hz), 2.78-2.70 (2H, m). 13C NMR (CDCl3) delta; 151.31, 149.00, 137.60, 137.37, 128.34, 128.16, 127.96, 127.84, 76.07, 75.77, 60.80, 60.71, 50.96, 50.62, 47.72, 44.41. HRMS: Calcd for C11H17N2O2 (M+H+) : 209.1289, Found : 209.1271. Anal. Calcd for C11H16N2O2 : C, 63.44; H, 7.74; N, 13.45, Found : C, 63.43; H, 7.76; N, 13.50.
N-(tert-Butoxycarbonyl)-N-(2-hydroxyethyl)aminoethanal O-Benzyloxime (3). To a solution of 2 (4.0 g, 19.2 mmol) in acetone (60 ml) was added a solution of Na2CO3 (3.5 g, 33 mmol) in H2O (15 ml) under a nitrogen atmosphere at rt. After di-tert-butyl dicarbonate (6.0 g, 28.8 mmol) was added dropwise at 0 deg.C, the reaction mixture was heated to reflux for 30 min. After the reaction mixture was filtered through a pad of Celite, the filtrate was concentrated at reduced pressure. The resulting residue was quenched with water and then extracted with CH2Cl2. The organic phase was washed with brine, dried over Na2SO4 and concentrated at reduced pressure. Purification of the residue by flash column chromatography (AcOEt/hexane 1:2) afforded 3 (5.4 g, 92%) as a colorless oil and a 3:2 mixture of E/Z-oxime. The presence of rotamers precluded a comprehensive assignment of all proton and carbon resonances. IR (CHCl3) 3435 (OH), 1689 (NCOO) cm-1. 1H NMR (CDCl3) delta; 7.45 (3/5H, br m), 7.38-7.25 (5H, m), 6.74 (2/5H, br m), 5.11 (4/5H, s), 5.06 (6/5H, s), 4.2-4.1 (4/5H, br m), 4.0-3.9 (6/5H, br m), 3.67 (2H, br m), 3.37 (2H, br m), 1.45 (18/5H, s), 1.44 (27/5H, s). 13C NMR (CDCl3) delta; 150.23, 147.88, 147.56, 137.48, 137.16, 128.31, 128.17, 127.94, 127.90, 127.84, 80.58, 76.12, 75.94, 61.46, 60.94, 50.73, 50.24, 47.36, 44.87, 28.22. HRMS: Calcd for C16H25N2O4 (M+H+) : 309.1812, Found : 309.1795.
N-(tert-Butoxycarbonyl)-N-(2-oxoethyl)aminoethanal O-Benzyloxime (4). To a solution of pyridine (15.7 ml, 195 mmol) in CH2Cl2 (250 ml) was portionwise added CrO3 (9.74 g, 97.4 mmol) under a nitrogen atmosphere at rt. After stirring at rt for 15 min, a solution of 3 (5.0 g, 16.2 mmol) in CH2Cl2 (32 ml) was added to the reaction mixture. After the reaction mixture was stirred at the same temperature for 1.5 h, the solvent was evaporated at reduced pressure. After the resulting residue was diluted with Et2O and filtered through a pad of Celite, the filtrate was concentrated at reduced pressure. Purification of the residue by flash column chromatography (AcOEt/hexane 1:3) afforded 4 (3.12 g, 63%) as a colorless oil and a 5:4 mixture of E/Z-oxime. The presence of rotamers precluded a comprehensive assignment of all proton and carbon resonances. IR (CHCl3) 1736 (CHO), 1698 (NCOO) cm-1. 1H NMR (CDCl3) delta; 9.51 (1H, br m), 7.41 (5/9H, t, J=5.1 Hz), 7.4-7.3 (5H, m), 6.82 (4/9H, t, J=4.4 Hz), 5.09 (8/9H, s), 5.05 (10/9H, s), 4.3-3.7 (4H, br m), 1.45 (36/9H, s), 1.42 (45/9H, s). 13C NMR (CDCl3) delta; 198.18, 197.59, 149.30, 148.66, 146.04, 137.28, 137.20, 128.41, 128.21, 128.12, 127.99, 81.34, 76.27, 75.98, 58.21, 56.67, 47.40, 46.68, 44.79, 44.14, 28.09. HRMS: Calcd for C16H23N2O4 (M+H+) : 307.1656, Found : 307.1653.
Radical cyclization using SmI2. To a solution of 4 (153 mg, 0.5 mmol) and tert-BuOH (0.12 ml, 1.25 mmol) in THF (10 ml) was added dropwise SmI2 (0.1 M in THF) (15 ml, 1.5 mmol) under an argon atmosphere at -78 deg.C. The reaction mixture was stirred at the same temperature for 30 min. After the solvent was evaporated at reduced pressure, the resulting residue was diluted with Et2O and filtered through a pad of Celite. After the filtrate was concentrated at reduced pressure, purification of the residue by flash column chromatography (AcOEt/hexane 2:1) afforded trans-5 (97 mg, 63%) and cis-5 (11 mg, 7%) both as a colorless oil. The presence of rotamers precluded a comprehensive assignment of all proton and carbon resonances.
trans-4-(Benzyloxyamino)-1-(tert-butoxycarbonyl)pyrrolidin-3-ol(trans-5). IR (CHCl3) 3600-3300 (OH, NH), 1675 (NCOO) cm-1. 1H NMR (CDCl3) delta; 7.4-7.3 (5H, m), 4.68 (2H, s), 4.23 (1H, br m), 3.65-3.55 (2H, br m), 3.53 (1H, br m), 3.3-3.1 (2H, br m), 1.45 (9H, s). 13C NMR (CDCl3) delta; 154.67, 137.27, 128.59, 128.47, 128.11, 79.62, 76.93, 72.61, 71.67, 66.45, 65.89, 51.91, 51.65, 47.44, 46.89, 28.44. HRMS: Calcd for C16H25N2O4 (M+H+) : 309.1768, Found : 309.1788.
cis-4-(Benzyloxyamino)-1-(tert-butoxycarbonyl)pyrrolidin-3-ol (cis-5). IR (CHCl3) 3600-3300 (OH, NH), 1689 (NCOO) cm-1. 1H NMR (CDCl3) delta; 7.4-7.3 (5H, m), 4.71 (2H, s), 4.25 (1H, br d, J=15.0 Hz), 3.7-3.4 (4H, br m), 3.04 (1H, br m), 1.44 (9H, s). 13C NMR (CDCl3) delta; 154.48, 137.20, 128.49, 128.17, 79.53, 76.57, 69.14, 68.37, 62.51, 61.82, 52.86, 52.58, 45.76, 45.07, 28.41. HRMS: Calcd for C16H24N2O4 (M+) : 308.1734, Found : 308.1746.
trans-4-Amino-1-(tert-butoxycarbonyl)pyrrolidin-3-ol (6). A suspension of PtO2 (50 mg, 0.22 mmol) in MeOH (1 ml) was stirred under a hydrogen atmosphere at rt for 1 h. A solution of trans-5 (100 mg, 0.32 mmol) in MeOH (3 ml) was added to a resulting suspension. After stirring under a hydrogen atmosphere at rt for 12 h, the reaction mixture was filtered and the filtrate was concentrated at reduced pressure. Purification of the residue by flash column chromatography (AcOEt/hexane 1:1) afforded 6 (50 mg, 76%) as a white solid. The presence of rotamers precluded a comprehensive assignment of all proton and carbon resonances. IR (CHCl3) 3600-3300 (OH, NH), 1690 (NCOO) cm-1. 1H NMR (CDCl3) delta; 3.98 (1H, br m), 3.69 (2H, br m), 3.29 (2H, br m), 3.10 (1H, br m), 1.46 (9H, s). 13C NMR (CDCl3) delta; 154.73, 79.60, 76.73, 76.20, 57.69, 57.11, 52.06, 51.67, 51.57, 51.32, 28.50. HRMS: Calcd for C9H18N2O3 (M+) : 202.1316, Found : 202.1340.
trans-1-(tert-Butoxycarbonyl)-4-[(3-methoxy-1-oxo-2-propenyl)amnocarbonyl-amino]pyrrolidin-3-ol (7a). To a solution of 6 (506 mg, 2.5 mmol) in DMF (3 ml) was added dropwise a solution of 3-methoxyacryloyl isocyanate16 (318 mg, 2.5 mmol) in benzene (3 ml) under a nitrogen atmosphere at -20 deg.C. After stirring at -15 deg.C for 1 hr, the reaction mixture was then stirred at rt for 18 h. After the solvent was evaporated at reduced pressure, the resulting residue was diluted with AcOEt. The organic phase was washed with water and brine, dried over Na2SO4 and concentrated at reduced pressure. Purification of the residue by flash column chromatography (CHCl3/MeOH 30:1) afforded 7a (747 mg, 91%) as a white powder. The presence of rotamers precluded a comprehensive assignment of all proton and carbon resonances. IR (CHCl3) 3600-3200 (OH, NH), 1687 (CO) cm-1. 1H NMR (CDCl3) delta; 9.27 (1H, br m), 8.92 (1H, br m), 7.69 (1H, d, J=12.2 Hz), 5.30 (1H, d, J=12.2 Hz), 4.3-4.1 (2H, br m), 3.75 (3H, s), 3.9-3.65 (3H, br m), 3.4-3.2 (2H, br m), 1.46 (9H, s). 13C NMR (CDCl3) delta; 168.13, 164.15, 155.87, 154.43, 97.05, 79.92, 74.87, 74.04, 57.97, 56.72, 56.24, 51.40, 50.96, 49.19, 48.56, 28.38. HRMS: Calcd for C14H24N3O6 (M+H+) : 330.1663, Found : 330.1666.
trans-1-(tert-Butoxycarbonyl)-4-[(3-methoxy-2-methyl-1-oxo-2-prpenyl)-aminocarbonylamino]pyrrolidin-3-ol (7b). Following the same procedure as for 7a, compound 7b was obtained from 6 and 3-methoxy-2-methylacryloyl isocyanate[16] in 53% yield as a white powder. The presence of rotamers precluded a comprehensive assignment of all proton and carbon resonances. IR (CHCl3) 3600-3200 (OH, NH), 1688 (NCOO) cm-1. 1H NMR (DMSO-d6) delta; 9.82 (1H, br s), 8.74 (1H, br m), 7.47 (1H, br s), 5.42 (1H, br d, J=3.8 Hz), 4.1-3.9 (2H, br m), 3.80 (3H, s), 3.54 (1H, br m), 3.34 (1H, br m), 3.13 (2H, m), 1.61 (3H, s), 1.40 (9H, s). 13C NMR (DMSO-d6) delta; 169.94, 158.55, 153.92, 153.77, 106.98, 78.71, 72.87, 72.03, 61.22, 55.64, 54.87, 51.79, 51.42, 49.66, 49.28, 28.21, 8.86. HRMS: Calcd for C15H25N3O6 (M+) : 343.1742, Found : 343.1746.
trans-4-(Uracil-1-yl)pyrrolidin-3-ol Hydrochioride (8a). A solution of 7a (300 mg, 0.91 mmol) in 2N-HCl (15 ml) was stirred at rt for 3 h. After the solvent was evaporated at reduced pressure, purification of the residue by washing with Et2O afforded 8a (180 mg, quant.) as a white powder. IR (Nujol) 3400-3200 (OH, NH), 1678 (CO) cm-1. 1H NMR (CD3OD) delta; 7.63 (1H, d, J=7.9 Hz), 5.71 (1H, d, J=7.9 Hz), 4.72 (1H, m), 4.54 (1H, m), 3.9-3.6 (2H, m), 3.55-3.2 (2H, m). 13C NMR (D2O) delta; 169.30, 154.72, 149.56, 104.95, 75.38, 70.20, 53.98, 49.17. HRMS: Calcd for C8H12N3O3 (M+H+) : 198.0878, Found : 198.0871.
trans-4-(Thymin-1-yl)pyrrolidin-3-ol Hydrochioride (8b). Following the same procedure as for 8a, compound 8b was obtained from 7b in quantitative yield as a white powder. IR (Nujol) 3400-3200 (OH, NH), 1698 (CO) cm-1. 1H NMR (D2O) delta; 7.49 (1H, s), 4.84 (1H, m), 4.62 (1H, m), 4.0-3.7 (3H, m), 3.37 (1H, dd, J=12.5, 5.6 Hz), 1.85 (3H, s). 13C NMR (D2O) delta; 169.46, 154.76, 145.05, 114.08, 75.43, 69.47, 53.94, 49.19, 14.23. HRMS: Calcd for C9H14N3O3 (M+H+) : 212.1034, Found : 212.1056.
trans-1-(tert-Butoxycarbonyl)-4-(6-chloro-9H-purin-9-yl)yrrolidin-3-ol (10). To a solution of 6 (162 mg, 0.8 mmol) in nBuOH (4.4 ml) was added 5-amino-4,6-dichloropyrimidine (164 mg, 1.0 mmol) and triethylamine (0.9 ml) under a nitrogen atmosphere at rt. After stirring at 120 deg.C for 14 h, the solvent was evaporated at reduced pressure. After the resulting residue was diluted with Et2O, a resulting powder was filtered to afford crude product 9. A solution of 9 in diethoxymethyl acetate (15 ml) was stirred under a nitrogen atmosphere at 110 deg.C for 7 h. After the solvent was evaporated at reduced pressure, a solution of p-TsOH.H2O (20 mg, 0.12 mmol) in toluene (30 ml) was added to the resulting residue under a nitrogen atmosphere at rt. After stirring at the same temperature for 2.5 h, the solvent was evaporated at reduced pressure. Purification of the residue by flash column chromatography (CHCl3/MeOH 20:1) afforded 10 (95 mg, 35%) as colorless crystals. The presence of rotamers precluded a comprehensive assignment of all proton and carbon resonances. mp 195-196 oC (AcOEt/hexane). IR (Nujol) 3300 (OH), 1674 (NCOO) cm-1. 1H NMR (CD3OD) delta; 8.77 (1H, s), 8.55 (1H, s), 5.04 (1H, br m), 4.84 (1H, br m), 4.06 (1H, br m), 3.97 (1H, br m), 3.83 (1H, br m), 3.34 (1H, br m), 1.50 (9H, s). 13C NMR (CD3OD) delta; 153.31, 152.91, 151.54, 146.93, 132.81, 127.54, 81.69, 73.48, 72.84, 62.83, 62.31, 52.35, 51.82, 48.78, 48.24, 28.72. HRMS: Calcd for C14H19N5O335Cl (M+H+) : 340.1175, Found : 340.1190. Anal. Calcd for C14H18N5O3Cl : C, 49.49; H, 5.34; N, 20.61; Cl, 10.43, Found : C, 49.36; H, 5.27; N, 20.54; Cl, 10.46.
trans-4-(Adenin-9-yl)-1-(tert-butoxycarbonyl)pyrrolidin-3-ol (11). A solution of 10 (900 mg, 2.65 mmol) in saturated methanolic ammonia (25 ml) in sealed tube was heated at 120 oC for 3 days. After the solvent was evaporated at reduced pressure, purification of the residue by flash column chromatography (CHCl3/MeOH 10:1) afforded 11 (806 mg, 95%) as a white powder. The presence of rotamers precluded a comprehensive assignment of all proton and carbon resonances. IR (Nujol) 3600-3200 (OH, NH), 1694 (NCOO) cm-1. 1H NMR (CD3OD) delta; 8.23 (1H, s), 8.05 (1H, s), 5.0-4.7 (2H, br m), 4.1-3.7 (3H, br m), 3.35 (1H, br m), 1.51 (9H, s). 13C NMR (DMSO-d6) delta; 156.26, 152.54, 149.72, 139.61, 119.30, 79.30, 79.03, 71.74, 70.94, 60.72, 59.49, 51.27, 50.81, 47.91, 47.44, 28.20. HRMS: Calcd for C14H20N6O3 (M+) : 320.1595, Found : 320.1606.
trans-4-(Adenin-9-yl)pyrrolidin-3-ol Trifluoroacetic acid (12). To a solution of 11 (750 mg, 2.34 mmol) in CH2Cl2 (1.7 ml) was added TFA (1.7 ml) under a nitrogen atmosphere at rt. After stirring at the same temperature for 24 h, the solvent was evaporated at reduced pressure. Purification of the residue by washing with Et2O afforded 12 (780 mg, quant.) as a white powder. IR (Nujol) 3600-3200 (OH, NH) cm-1. 1H NMR (D2O) delta; 8.37 (2H, br s), 5.26 (1H, br m), 4.81 (1H, br m), 4.08 (2H, m), 3.83 (1H, dd, J=12.8, 5.1 Hz), 3.49 (1H, dd, J=12.8, 3.2 Hz). 13C NMR (D2O) delta; 152.90, 151.01, 147.31, 147.02, 142.80, 76.71, 64.60, 53.89, 49.72. HRMS: Calcd for C9H13N6O (M+H+) : 221.1168, Found : 221.1159.
(6) Acknowledgements
We wish to thank the Ministry of Education, Science, Sports and Culture of Japan and the Science Research Promotion Fund of the Japan Private School Promotion Foundation for research grants.
(7) References and notes
[1] For reviews, see: a) Huryn, D. M.; Okabe, M. Chem. Rev. 1992, 92, 1745-1768. b) Borthwick, A. D.; Biggadike, K. Tetrahedron 1992, 48, 571-623.
[2] For examples, see: a) Deng, L.; Schårer, O. D.; Verdine, G. L. J. Am. Chem. Soc. 1997, 119, 7865-7866. b) Leggio, A.; Liguori, A.; Maiuolo, L.; Napoli, A.; Procopio, A.; Siciliano, C.; Sindona, G. J. Chem. Soc., Perkin Trans. 1 1997, 3097-3099. c) Lowe, G.; Vilaivan, T. J. Chem. Soc., Perkin Trans. 1 1997, 539-546. d) Peterson, M. L.; Vince, R. J. Med. Chem. 1991, 34, 2787-2797. e) Harnden, M. R.; Jarvest, R. L. Tetrahedron Lett. 1991, 32, 3863-3866. f) Mansour, T. S.; Jin, H. Bioorg. Med. Chem. Lett. 1991, 1, 757-760. g) Ng, K.-M. E.; Orgel, L. E. J. Med. Chem. 1989, 32, 1754-1757. h) Kaspersen, F. M.; Pandit, U. K. J. Chem. Soc., Perkin 1 1975, 1617-1622 and 1798-1802.
[3] a) Jacobson, K. A.; van Galen, P. J. M.; Williams, M. J. Med. Chem. 1992, 35, 407-422. b) Wolfe, M. S.; Borchardt, R. T. J. Med. Chem. 1991, 34, 1521-1530.
[4] For recent reviews, see: a) Ryu, I.; Sonoda, N.; Curran. D. P. Chem. Rev. 1996, 96, 177-194. b) Snider, B. B. Chem. Rev. 1996, 96, 339-364. c) Giese, B.; Kopping, B.; Göbel, T.; Dickhaut, J.; Thoma, G.; Kulicke, K. J.; Trach, F. Org. React. (N.Y.) 1996, 48, 301-856.
[5] Fossey, J.; Lefort, D.; Sorba, J. Free Radicals in Organic Chemistry.; Translated by Lomas, J., John Wiley & Sons: New York, 1995.
[6] For our examples of the reaction of oxime ethers with ketyl radical or alkyl radical, see: a) Miyabe, H.; Shibata, R.; Ushiro, C.; Naito, T. Tetrahedron Lett. 1998, 39, 631-634. b) Miyabe, H.; Ushiro, C.; Naito, T. Chem. Commun. 1997, 1789-1790. c) Miyabe, H.; Torieda, M.; Kiguchi, T.; Naito, T. Synlett 1997, 580-582. d) Naito, T.; Torieda, M.; Tajiri, K.; Ninomiya, I.; Kiguchi, T. Chem. Pharm. Bull. 1996, 44, 624-626.
[7] For recent examples of the radical reaction of oxime ethers, see: a) Clive, D. L. J.; Zhang, J. Chem. Commun. 1997, 549-550. b) Marco-Contelles, J.; Balme, G.; Bouyssi, D.; Destabel, C.; Henriet-Bernard, C. D.; Grimaldi, J.; Hatem, J. M. J. Org. Chem. 1997, 62, 1202-1209. c) Kim, S.; Lee, I. Y.; Yoon, J.-Y.; Oh, D. H. J. Am. Chem. Soc. 1996, 118, 5138-5139. d) Keck, G. E.; Wager, T. T. J. Org. Chem. 1996, 61, 8366-8367. e) Bhat, B.; Swayze, E. E.; Wheeler, P.; Dimock, S.; Perbost, M.; Sanghvi, Y. S. J. Org. Chem. 1996, 61, 8186-8199. f) Hollingworth, G. J.; Pattenden, G.; Schulz, D. J. Aust. J. Chem. 1995, 48, 381-399. g) Chiara, J. L.; Marco-Contelles, J.; Khiar, N.; Gallego, P.; Destabel, C.; Bernabé, M. J. Org. Chem. 1995, 60, 6010-6011. h) Santagostino, M.; Kilburn, J. D. Tetrahedron Lett. 1995, 36, 1365-1368.
[8] For the first example of the radical reaction of oxime ethers with O-stannylketyl radical, see: a) Naito, T.; Tajiri, K.; Harimoto, T.; Ninomiya, I.; Kiguchi, T. Tetrahedron Lett. 1994, 35, 2205-2206. b) Kiguchi, T.; Tajiri, K.; Ninomiya, I.; Naito, T.; Hiramatsu, H. Tetrahedron Lett. 1995, 36, 253-256.
[9] Stach, L. J. U. S. Patent 3920772, 1975 (Chem. Abstr. 1976, 84, 89603d).
[10] The E/Z ratios were determined by 1H-NMR spectroscopy. In general, the downfield shifts of signals due to the imino hydrogen of the E-oxime ether are observed by the influence of the neighboring alkoxy group on the oxime ether moiety. See: McCarty, C. G. The Chemistry of Functional Groups; The chemistry of the carbon-nitrogen double bond; Patai, S., Ed., John Wiley & Sons: New York, 1970; pp.383-392.
[11] The stannyl radical-induced radical cyclization of 4 gave a 3:1 mixture of trans-5 and cis-5 in 66% combined yield.
[12] For reviews, see: (a) Skrydstrup, T. Angew. Chem., Int. Ed. Engl. 1997, 36, 345-347. (b) Molander, G. A.; Harris, C. R. Chem. Rev. 1996, 96, 307-338.
[13] We have observed no remarkable effect of the geometry of the starting oxime ether group on either the chemical yield or trans/cis selectivity of radical cyclization product.
[14] Previously, we reported that the addition of HMPA, which was recognized to increase the reaction rate by SmI2, was found to be crucial for successful seven-membered ring formation. See: reference 3c.
[15] For selected examples of the reductive coupling of oxime ether and carbonyl compound by use of SmI2, see: a) Marco-Contelles, J.; Gallego, P.; Rodríguez-Fernández, M.; Khiar, N.; Destabel, C.; Bernabé, M.; Martínez-Grau, A.; Chiara, J. L. J. Org. Chem. 1997, 62, 7397-7412. b) Hanamoto, T.; Inanaga, J. Tetrahedron Lett. 1991, 32, 3555-3556.
[16] Shaw, G.; Warrener, R. N. J. Chem. Soc. 1958, 153-156 and 157-161.