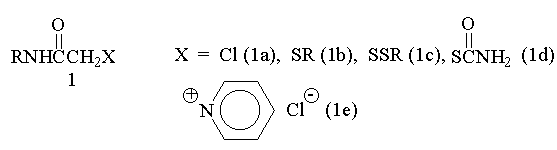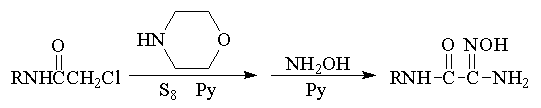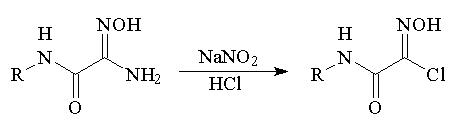Synthesis of Amido-Containing Heterocyclic Compounds
V.N. Yarovenko,* S.A. Kosarev, I.V. Zavarzin, and M.M. Krayushkin.
N.D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 47 Leninsky prosp., 117913 Moscow, Russian Federation
Abstract: A convenient
approach to the synthesis of amido-containing 1,3,4-oxadiazoles,
tetrazoles, isoxazolines, isoxazoles, and oxathiazoles was
suggested.
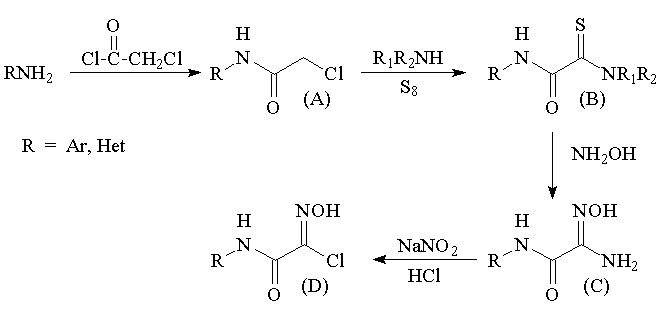
We suggested an
approach to the synthesis of several amido-containing heterocyclic
compounds using heterocyclization of carbamoylhydroxymoyl chloride
fragments (D) obtained by the successive modification of accessible
chaloracetamide groups according to Scheme 1.
Scheme 1
(D) R=
Ph
Monothiooxamides
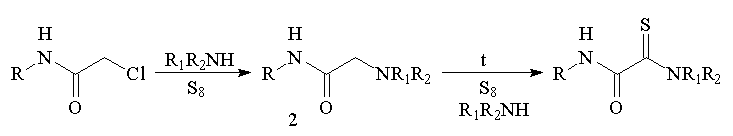
(B) are formed in the reactions of different a-substituted
derivatives of acetamide 1 with preliminarily prepared
solutions of elemental sulfur in amines. a-Chloracetamides
(1a) and their pyridinium salts (1e) react most
smoothly.
A possibility to
obtain monothiooxamides by the reactions of chloracetamides with
sulfur and amines has been previously shown in the literature using
several examples. However, no preliminarily prepared solutions of
sulfur in amines were used in these processes, and a high temperature
was used in the reactions. Our attempts to obtain several
monothiooxamides by the method described resulted in the synthesis of
the target products only in insignificant amounts. We showed that
when sulfur and amine are simultaneously added to chloracetamides, an
aminomethylene derivative (2) is mainly formed and then it can
react with sulfur only at a high temperature.
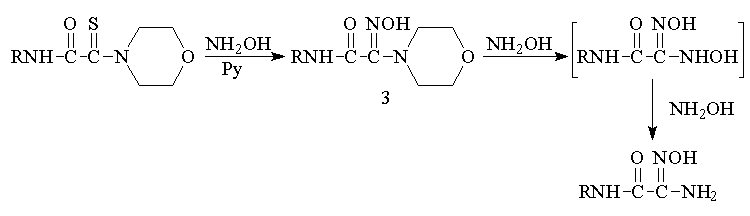
We showed that
the reactions of monothiooxamides in pyridine with an excess of
hydroxylamine, which plays the role of the reducing agent in this
reaction as well, afford carbamoylamidoximes in high yields. The
reaction proceeds via the stage of formation of substituted
amidoxime 3, which can be isolated in high yield when the
reaction is carried out at room temperature
It is noteworthy
that the synthesis of nonsubstituted amidoximes from N(S)-substituted
thioamides has not been described previously.
We also showed
that carbamoylamidoximes can be synthesized in sufficiently high
yields in one flask by the reaction of the corresponding
chloracetamide with elemental sulfur and amine in pyridine followed
by the treatment of the reaction mass with hydrochloric hydroxylamine
without intermediate isolation of the monothiooxamide
formed.
We established
that when hydrochloric acid is added to an aqueous suspension of
carbomoylamidoximes, water-soluble salts are formed, which are
rapidly transformed under the action of sodium nitrite at room
temperature into hydroxymoyl chlorides.
The reactions of
hydroxymoyl chlorides with different nucleophiles make it possible to
synthesize different heterocyclic compounds.
Thus, we
developed an approach, which allows one to synthesize different
amido-containing heterocyclic compounds by several simple
modifications of amino group.