The analysis of the electron density as introduced by Bader gives a
mathematically stringent definition of a chemical bond.
The existence of a bond is indicated by the presence of a so-called
bond critical point (bcp).
This is a saddle point (
![]() )
in the electron density
between two nuclei, where the Hessian (the second derivative matrix) of the
electron density has one positive eigenvalue in the bond axis and two negative
eigenvalues perpendicular to the bond axis. From this follows that there exists
a path of maximum electron density from one nucleus to another passing through
the bond critical point [15]. This path is called ``the bond''.
)
in the electron density
between two nuclei, where the Hessian (the second derivative matrix) of the
electron density has one positive eigenvalue in the bond axis and two negative
eigenvalues perpendicular to the bond axis. From this follows that there exists
a path of maximum electron density from one nucleus to another passing through
the bond critical point [15]. This path is called ``the bond''.
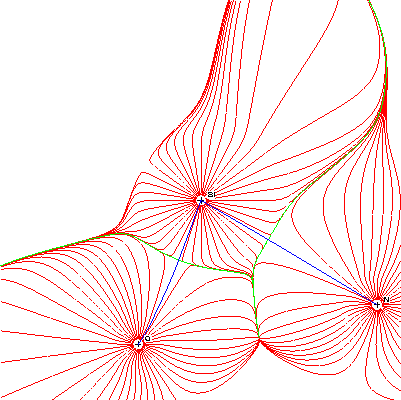
We determined some bond critical points and bond paths for our chiral silatrane. The bond paths are visualised in figure 4 and the properties of the critical points are summarised in table 5. Figure 4 shows clearly the bond path between silicon and nitrogen, as well as the path between silicon and oxygen. The bond critical points are the crossing points of the bond path (blue) and the interatomic surface (zero-flux surface, green) (for a definition, see below).
 |