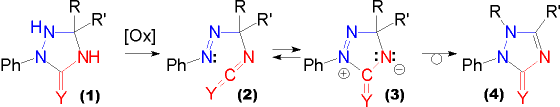
| Upon oxidation triazolidin-3-ones (1, Y = O) as well as triazolidine-3-thiones (1, Y = S) undergo an oxidative ring opening to yield gem azoalkylisocyanates (2, Y = O) and -isothiocyanates (2, Y = S), respectively. These species can be conceived as being in equilibrium with the zwitterionic cyclic valence tautomer (3). |
Originating from this tautomer thermal or acid induced rearrangement to triazolin-3-ones (4, Y = O) and -3-thiones (4, Y = S) occurs, i.e. migration of a substituent from ring position 5 to ring position 1. The migratory aptitude in this case corresponds to the ability of the migrating group to accomodate a positive charge. |