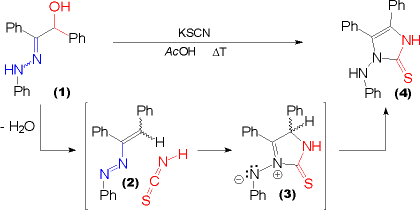

| With thiocyanic acid benzoin phenylhydrazone (1) forms the imidazolinethione (4). The mechanistic interpretation of this reaction starts with the conjugated 1,4-elimination of water from the hydrazone (1) to give the azoalkene intermediate (2). These compounds are known to react with thiocyanic acid forming an azomethine imine (3).1 | This, in turn, tautomerizes to the imidazolinethione (4) obtained. The typical METHOD FOR THE SYNTHESIS OF IMIDAZOLINETHIONES of this type involves a-haloketones. While the conversion of desylchloride failed to give the imidazolinethione (4), the conversion of (1) into (4) was successful. |
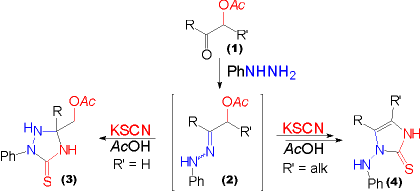
| When a-acetoxyketones (1) with the acetoxy group attached to a secondary carbon center are subjected to the "one pot procedure", imidazolinethiones (4) were obtained, analogously to the reaction of a-haloketones. By contrast, triazolidine-3-thiones (3) are formed when the acetoxy group is attached to a primary a-carbon atom. Again, the mechanistic rational starts from an acetoxyphenylhydrazone (2) formed in a first step from the ketone and phenylhydrazine. |
Acetic acid is then eliminated to yield an azoalkene as outlined as GENERAL CASE OF IMIDAZOLINETHIONE SYNTHESIS. A mechanistic borderline between two related reaction paths is encountered in this reaction crossroad - triazolidine-3-thione formation and formation of imidazolinethiones via azoalkene intermediates. The formation of the azoalkene is favored by the higher substitution of the C-C double bond in the case of acetoxy groups attached to secondary or BENZYLIC ("BENZOIN CASE") carbon atoms. |
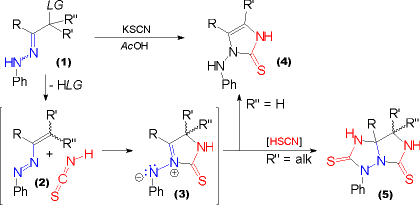
| The usual methodology for the preparation of imidazolinethiones with a 1-anilino substituent starts from a-haloketones where the halogen atom is attached to a secondary carbon atom. When subjected to the one pot procedure using phenylhydrazine and potassium thiocyanate in acetic acid an unstable a-halophenylhdrazone (1, LG = halogen) is formed in a first step. This eliminates HLG (= HX in this case) very easily to give rise to an azoalkene (2). | This, in turn, adds a molecule of thiocyanic acid in the course of a dipolar [3+2] cycloaddition. Thus an azomethine imine (3) forms. When R'' = H tautomerization occurs leading to the formation of the imidazolinethione (4) with a 1-anilino substituent. When R'' = alkyl tautomerization cannot occur and a second molecule of thiocyanic acid adds as a dipolarophile to the azomethine imine (3) to yield the heterobicyclic imidazotriazolidinethiones (5) shown. |