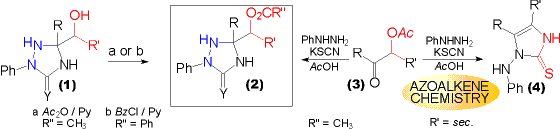
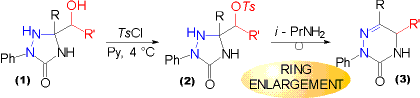
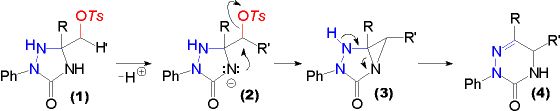
| Hydroxyalkyl substituted triazolidin-3-ones (2, Y = O) and -thiones (2, Y = S) can be acetylated (R'' = CH3) by the action of acetic acid anhydride, and benzoylated (R'' = Ph) with benzoylchloride. Care must be taken in the case of triazolidine-3-thiones (2, Y = S), as they are susceptible to a second benzoylation at ring position 4 in alkaline medium (Schotten Baumann conditions).
|
The resulting acetyloxy substituted triazolidine-3-thiones (2, R'' = CH3, Y = S) are also accessible directly from a-acetoxyketones using a one pot procedure. Nevertheless this method is confined to substrates, where the acetoxy group is attached to a primary carbon centre. If attached to a secondary one, the products obtained are derived from reactions involving INTERMEDIATE AZOALKENES.
|