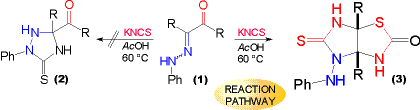
R = Me, Ph

| On treatment of a-oxophenylhydrazones (1) with potassium thiocyanate in acetic acid the expected acyl substituted triazolidine-3-thiones (2) were not formed. At elevated temperatures new heterobicyclic imidazothiazoles (3) were obtained instead (the structure of which is evidenced by X-ray). | Again an imidazolidinethione moiety with an aniline substituent is found in the molecule, parallelling the chemistry of azoalkene intermediates. On this basis, a REACTION PATHWAY for the formation of this new heterobicyclic structure is put forth and may be discussed. |
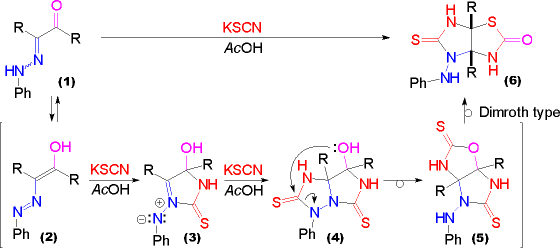
| The first central point in the proposed reaction pathway is the tautomeric equilibrium of the a-oxohydrazone (1) with a corresponding azoenol species (2). An azoalkene is expected to be susceptible to the cycloaddition reaction of thiocyanic acid to form an azomethine imine (3). | In contrast to SIMILAR REACTIONS AND REACTION PATHWAYS a further cycloaddition reaction of thiocyanic acid does not give an isolable imidazotriazolidinone (4), though it may be formed as an intermediate. Opening of the triazolidinone ring by the hydroxy group in (4) to form the oxazole (5) is followed by a Dimroth type rearrangement to furnish the product (6). |
| TOPICS OF: | C) OXOPHENYLHYDRAZONES |
| C1) New Heterobicyclic Systems C1.1) Reaction Pathway C1.2) Compare to: Azoalkene Chemistry NEXT CHAPTER HOME INDEX |