| [Related articles/posters: 110 018 119 ] |
Earlier [1], we reported that isoxazoles (I) reacted with arenethioles in polar aproton dissolvers in the presence of K2CO3 or another bases. In this paper, we describe the reactions of quinones (I), (II) with 8-mercaptoquinoline. These reactions (I ® III, II ® IV) have been conducted in conditions, similar to that described [1]. It has been found, that 8-mercaptoquinolines react as S-nucleophiles in these reactions:
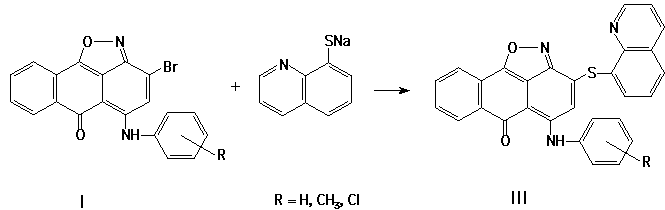
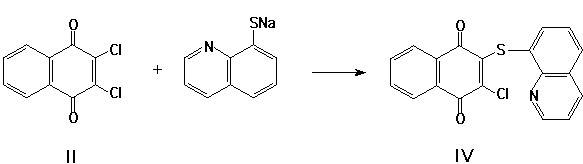
The cycles obtained (III, IV) are of self-dependent interest and may be applied as intermediates. The variants of thermal transformations of compounds (III) [2-3] may be as following:
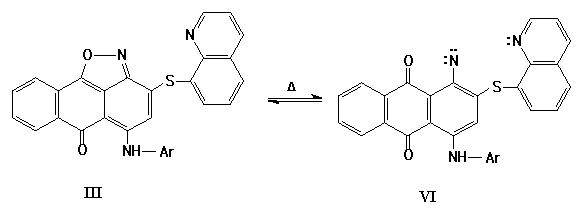
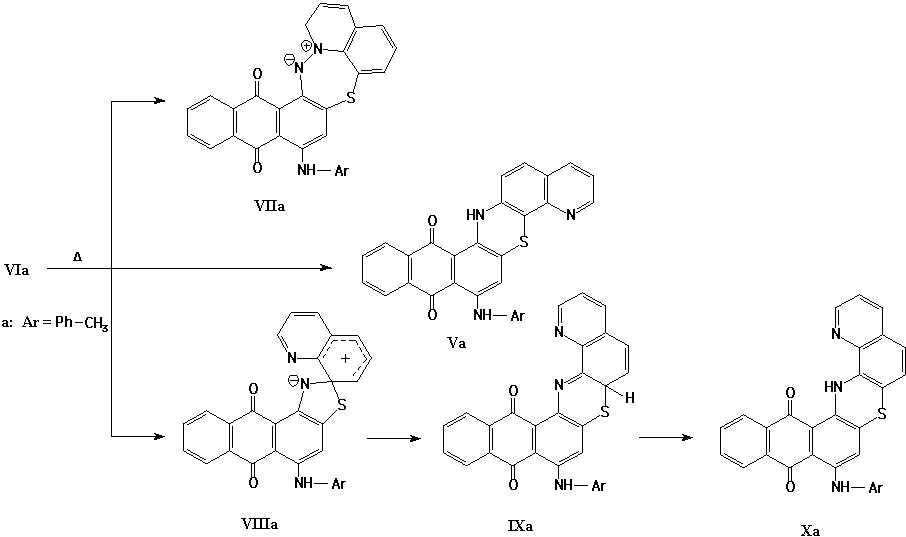
The compound VIIa may be obtained as a result of intramolecular reaction of nitrene (VIa). The products (Va), (Xa) may be obtained either as a result of direct insertion of nitrene (VIa) in aromatic C-H bond or during the rearrangement of the intermediate (VIIIa).
The electronic absorption spectrum of the compound, which was obtained in the course of thermolysis of isoxazolone (IIIa), is almost identical to the absorption spectrum of 6-p-toluidinonaphtho[2,3-a]phenothiazine-7,12-dione (XI) [Fig. 1]:
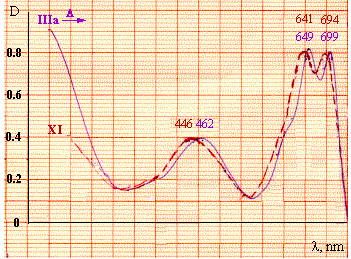
Fig. 1 Electronic absorption spectra of the product of isoxazolone (IIIa) thermolysis and 6-p-toluidinonaphtho[2,3-a]phenothiazine-7,12-dione (XI)
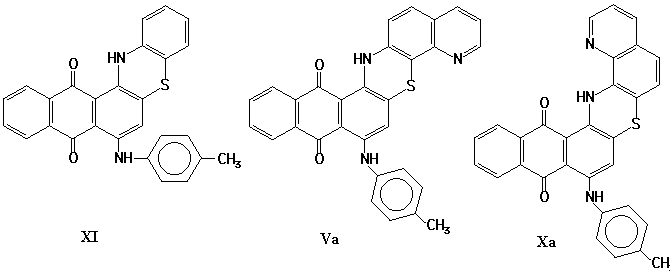
Thus, the structures either Va or Xa may be ascribed to the product of isomerization of the isoxazolone IIIa. The exact structure of the product will be ascertained later on.
Experimental
UV and VIS spectra were recorded on a Specord UV-VIS spectrophotometer (toluene as solvent). Melting temperature was measured on a Boetius melting-point apparatus. The data of elemental analysis of the synthesized compounds are equal to that calculated.
Syntheses
3-chloro-1,4-naphthoquinonyl-2,8'-quinolinylsulphide (IV)
12.19 g (5.5 mmol) of sodium 8-mercaptoquinolinate was added to a solution of II (2.3 g, 10 mmol) in DMAA (10 ml) and then stirred for 3 h at 20-25 žC. The solution was cooled off, the precipitate of IV was filtered and washed with water. Yield 2.8 g (78.65 %), mp 156 žC.
3-(8-quinolinylthio)-5-toluidino-6-oxo-6H-anthra[1,9-cd]isoxazole (IIIa)
Sodium 8-mercaptoquinolinate (0.36 g, 1.9 mmol) and potassium carbonate (0.2 g, 1.7 mmol) were added to a solution of I (0.4 g, 0.98 mmol) in DMFA (5 ml) and stirred for 3-4 h at 20-25 žC. The solution was cooled off, the precipitate of III was filtered and washed with water.
Yield 0.43 g (89.77 %). Brown crystals. mp ~ 200 žC (with decomposition).
Thermolysis of IIIa
3-(8-quinolinylthio)-5-toluidino-6-oxo-6H-anthra[1,9-cd]isoxazole (IIIa) (0.25 g, 0.5 mmol) in 3 ml of o-dichlorobenzene was boiled for 20-25 min. Then the solution was cooled off, the precipitate of the product was filtered and washed with ether. Yield 0.22 g (88 %). Green crystals. mp > 300 žC.
Acknowledgements
This work has been done under the financial support of Krasnoyarsk Regional Science Foundation, grant №: 7F0187
References