| [Related articles/posters: 119 110 074 ] |
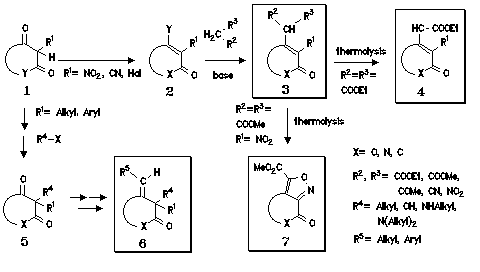
The thermolytic degradation of ethyl malonates of type 3 gives the ethyl acetates 4. With the 2-quinolone ring system this class of compounds shows manifold pharmacological activities [2].
Methyl malonates 3 having as substituent R1 a nitro group form under thermolytic conditions by loss of carbon dioxide and methanol isoxazoles of type 7. The thermolysis reactions to 4 and 7 have been studied by differential scanning calorimetry (DSC) [3].
The Wittig- or Grignard reaction of 2,2-disubstituted 1,3-dicarbonyl carbo- or heterocycles 5 with alkyl-triphenylphosphonium halides leads to 1-alkylidene-3-oxo compounds 6. This compound class was recently found to show antiviral activities [4].
[2] Z. Y. Jiang, Q.-L. Zhou, J. W. Eaton, W. H. Koppenol, J. V. Hunt, S. P. Wolff, Biochem. Pharmacol. 42, (1991) 1273; W. Carling, P. D. Leeson, K. W. Moore, M. Rowley (Merck, Sharp and Dohme Ltd), PCT Int. Appl. 93 11, 114 (1993).
[3] Th. Kappe and W. Stadlbauer, Molecules 1 (1996) 255.
[4] R. Kirsch, J. P. Kleim, G. Riess, M. Rösner, I. Winkler (Hoechst AG), Eur. Pat. EP 0 697 405 A1 (1996).