| [Molecules: 5] [Related articles/posters: 103 037 040 002 046 ] |
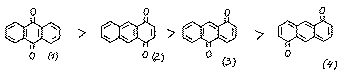
The quinones (3-4) are not described. For the derivatives of quinone (3), quantum chemical calculations show [2] that the activity of each position with respect to nucleophiles decreases: 9>4>2. Experimental data prove this. [3]
6H-6-oxoanthra[1,9-cd]isoxazoles (5) are heteroanalogues of 1,10-anthraquinones. For isoxazoles (5) the nucleophilic substitutions are readily carried out:[4-6]
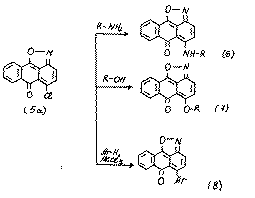
In the given reactions (5-8) the isoxazolic cycle is not destroyed.But the boiling of substances (6) in DMFA leads to regenerative splitting of the isoxazolic cycle:
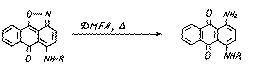
(6)
Anthra[1,9;-5,10-cd;d'c'] diisoxazole is a stable heteroanalogue of the unknown 1,5-anthraquinone (4):

It is expected that the positions 9,10 in substrate (9) must be the most active towards nucleophiles. We have studied the reactivity of substrate (9) towards various amines and found that at 20-25 oC. diisoxazole (9) reacts with amines in polar aprotic solvents or in amine media with the production of a multicomponent mixture of coloured products of a quinoid character. Their chromotographic division is difficult because of the low stability of the given compounds on silica gel.
Using column chromotography we separated the final products of the amination of isoxazole (9):
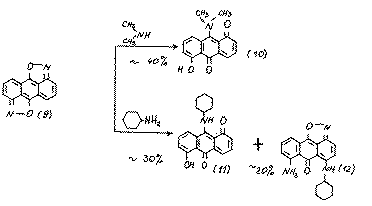
The structures of compounds10 and 11 was proved using mass spectrometric data and their chemical properties. For example, during their hydrolysis we obtained 1,5-dihydroxy-9,10-anthraquinone, which proves the presence of an amino group at position 9:
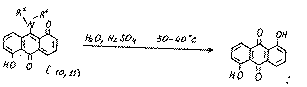
The formation of compounds 10 and 11 may be explained by the realization of the following approximate scheme:
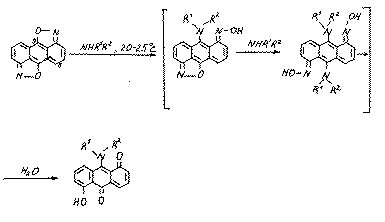
It is obvious,that because of the steric difficulties the substrate (9) may also be attacked by the cyclogexilamine at position 5:
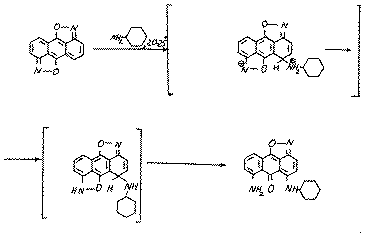
The reactions,we have found, prove the high reaction ability of diisoxazole (9) and the ability of the isoxazolic cycle to be split. The reactions result in the stable derivatives of 1,10-anthraquinone (10, 11).