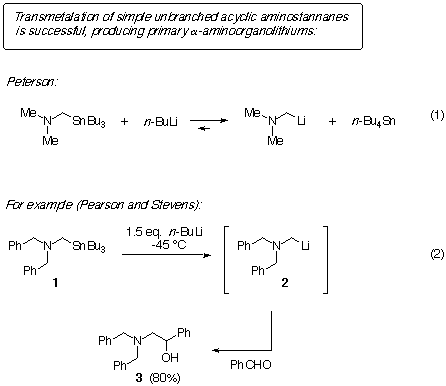

We found that the transmetalation of branched stannanes such as 4
and 6 was unsuccessful in generating the secondary a-aminoorganolithiums
5 and 7, respectively (eqns. 3 and 4). Even under drastic conditions,
starting material was recovered in good yield. Click
here if you want to see how we made the stannane starting materials.
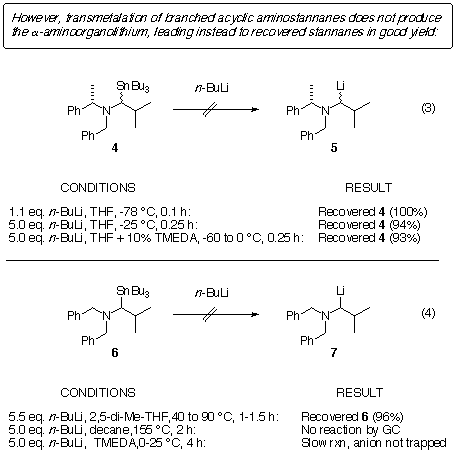
Others have reported similar results. Tsunoda and coworkers did not
observe tin-lithium exchange in eqn. 5, although they could generate this
organolithium by reductive cleavage of an organosulfur compound [4]. Hence, there
is nothing inherently wrong with these organolithiums; tin-lithium exchange
is just not the way to go about making them. Chong and coworkers [5] were unable to transmetalate the stannane shown in eqn. (6). Pearson
and Stevens (this work) found that recovered starting material was isolated
from attempted transmetalations of 8. From the examples in eqns. (5)
and (7), it is apparent that stannanes bearing an adjacent cyclic amino group
fared no better than those with an acylic amino group in transmetalations.
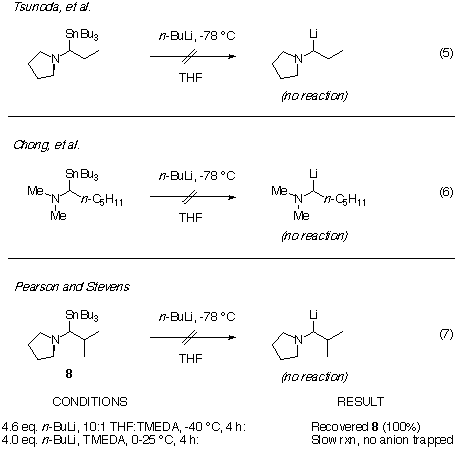
Does tin-lithium exchange fail because the alkyllithium reagent (e.g.
BuLi) does not attack the tin atom for some reason? The answer
is no. We have found that while the attempted transmetalation of the trimethylstannyl
compound 9 fails, the methyl groups are replaced by butyl
groups, thus proving the intermediacy of the stannate 11. Tin-lithium
exchange is well-known to be an equilibrium process, with the most stable
organolithium being formed preferentially. This experiment is a key one,
showing that the desired a-aminoorganolithium is less stable than
both methyl- and butyl-lithium! Alternatively, there may be some stereoelectronic
reason that the desired a-aminoorganolithium does not dissociate from
the stannate. We will return to this point in the Hypothesis
Section.
You may be wondering if BusLi or tert-butyllithium would force
the transmetalation. They do not. The stannanes are untouched by these reagents,
presumably due to steric reasons.
From the failure to transmetalate branched acyclic aminostannanes, one might conclude that secondary a-aminoorganolithiums are inaccessible by this method. Not so! Read on.