| [Molecules: 3] [Related articles/posters: 056 062 074 101 002 ] |
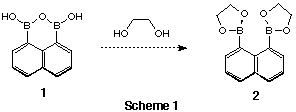
We planned to synthesiss 2 as a chelate Lewis acid by the reaction
of 1 with ethane-1,2-diol. In general, the boronic anhyride moiety
is easily reacted with alcohols to give esters.[3] Compound 1 and ethane-1,2-diol
were expected to react at the boronic anhyride moiety to give 1,3,2-dioxaborolane
groups (Scheme 1). The reaction with ethane-1,2-diol in toluene under reflux
gave surprisingly the stable 14-membered cyclic ester which is barely
soluble in most organic solvents. The structure of 3 was detemined
by X-ray single cystallolgraphy 1H and 13C NMR spectra. The cystal structure
of 3 is shown below. The ethylene bridges rotate freely in
solvent (seen by low temperature 1H NMR in toluene). In this reaction
boronic anhyride was very stable to diols because the geometry of the two boron
atoms was fixed to the naphthalene ring.
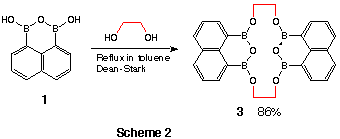
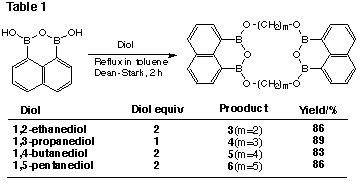
Reaction with a variety of a,omega-diols gave macrocyclic boronic anhydride
esters consisting of two moieties of the boronic anhydride and the diol (Table
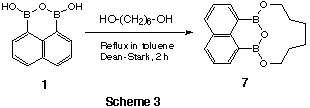
1). On the other hand, reaction with hexane-1,6-diol gave an 11-membered cyclic ester
7 including one diol and boronic anhyderide moiety in a single molecure (Scheme
3).
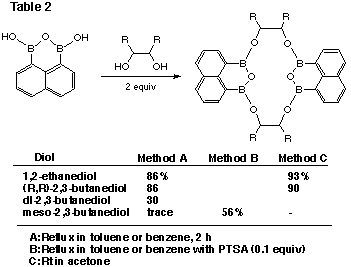
We carried out the reaction with symmetric 1,2-diols by three methods (Table
2).
Reactions with dl-, (R,R)-, mesobutane-2,3-diol
afford the corresponding cyclic esters as single stereoisomers in each case.
The fact that different isomers have been formed from the dl- and (R,R)-alcohols
indicates that the product formed from dl-alcohol contains the (R,R)-
and (S,S)-unit in the same molecure.
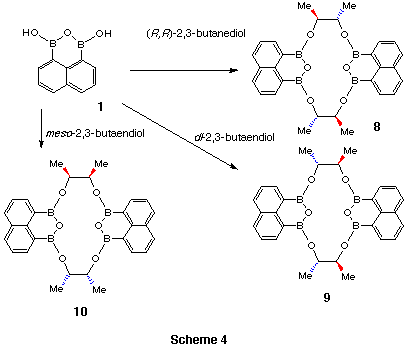
Conformational analysis of these butane-2,3-diol products are based on the X-ray single crystallography, 1H- and 13C NMR spectra, and semiempirical molecular orbital calculations. The confomation of (R,R)-butane-2,3-diol product was detemined by X-ray single crystallography (model 8). This conformation was in contrast to 3 because of the demand for arranging all methyl groups outside the ethylene bridges. The dl-isomer product xx must be the same as 3 for the same reason. The structure of meso isomer could not be determined by this rule. The most stable confomer was found by semiempirical molecular obital calculations.