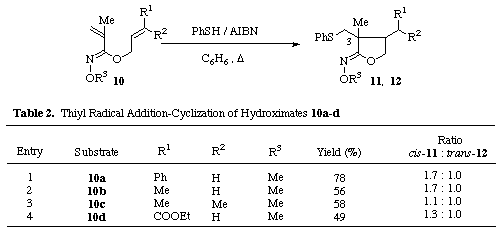
Expecting that, contrary to the esters, Z-O-alkylhydroximates would exist in a conformer A preferable to intramolecular cyclization over the less favored conformer B due to the steric repulsion between the substituents on nitrogen and oxygen atoms in the conformer B, we first investigated the radical cyclization of allyl Z-O-methylcinnamoylhydroximate 4a [5] in the presence of thiophenol and AIBN (Table 1, entry 1). A solution containing thiophenol (1 equiv.) and AIBN (0.5 equiv.) in benzene was added dropwise by a syringe pump over 2 h to a solution of 4a in boiling benzene while stirring under nitrogen. The solution was then refluxed for further 1 h and the solvent was removed in vacuo. The resulting residue was purified by medium pressure column chromatography to give a 1.8 : 1 mixture of the cis-5a [6] and trans-cyclized products 6a [6] in 82% combined yield.

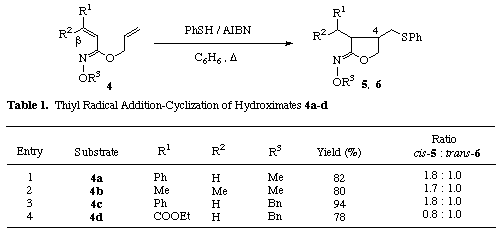
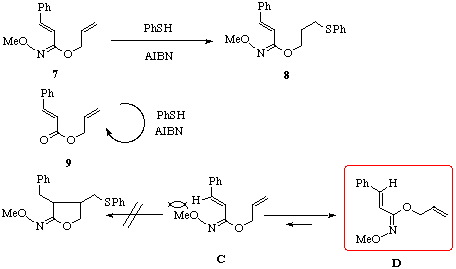
Under the same reaction condition, E-isomer 7 [5] and the corresponding ester 9 did not give the cyclized products and the substrates were recovered though E-hydroximate 7 gave an adduct 8 in 3% yield. The fact that E-O-alkylhydroximate 7 did not cyclize would be explained as follows. E-Isomer 7 would exist in a stable conformer D which is unfavorable to intramolecular cyclization. Another conformer C is preferable to cyclization but has the steric repulsion between methoxy group and olefinic hydrogen.
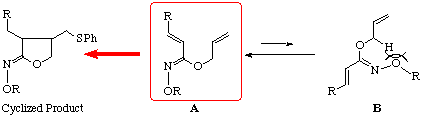
The substituent effect of the thiyl radical addition-cyclization was then investigated in order to establish the scope and limitations as shown in Table 1. The hydroximates 4b-d [5] with substituents at b-position of the unsaturated hydroximate group gave the desired cyclic hydroximates 5b-d [6] and 6b-d [6]in good yields which possess the phenylthiomethyl group at 4-position. Alternatively, the absence of the substituents at b-position in the unsaturated hydroximates 10a-d [5] influenced markedly the regioselectivity of the addition of the phenylthiyl radical, resulting in the exclusive formation of the 3-phenylthiomethyl products 11a-d [6] and 12a-d [6] as shown in Table 2. These results suggest that the dienes connected with Z-hydroximates undergo smooth 5-exo-trig type of radical cyclization though its regiochemistry depends on the substituents attached to the ¼-bond.