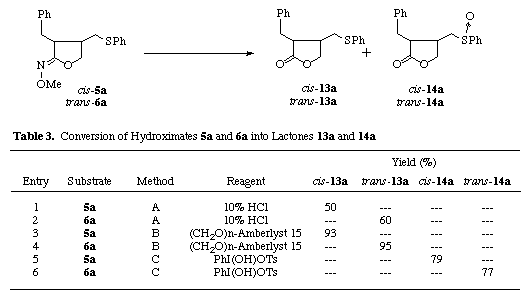
Conversion of the cyclic hydroximates to the lactones was very readily achieved as follows (Table 3). To our knowledge, there is no example of the transformation of the hydroximates to the esters or lactones. Hydrolysis of cis-5a and trans-6a with paraformaldehyde and Amberlyst [7] or 10% HCl gave the desired cis- and trans-lactones 13a in 50-60 % or 93-95 % yield (entries 1-4). The cis- and trans-lactones 14a having a sulfinyl group can be easily generated from the cyclic hydroximates cis-5a and trans-6a upon oxidation with hypervalent organoiodo reagent, [hydroxy(tosyloxy)iodo]benzene, in 77-79 % yield (entries 5, 6).
