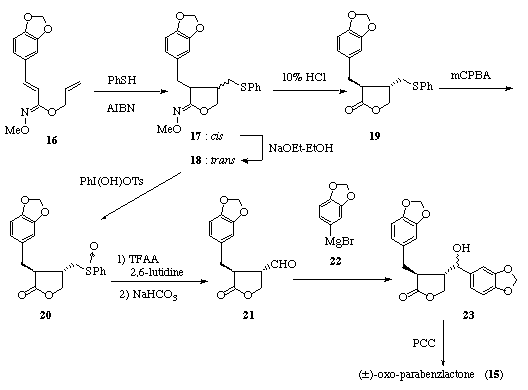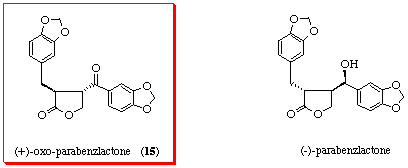
A new lignan of the dibenzylbutyrolactone type, (+)-oxo-parabenzlactone (15), [8] was recently isolated from the wood of Protium tenuifolium (Burseraceae). Previously, the enantiomer of 15 was obtained as an oxidation product of a lignan, (-)-parabenzlactone, which had been isolated from Parabenzoin trilobum Nakai. [9] [10] The lignans of the dibenzylbutyrolactone type exhibit various biological activities such as antitumor and platelet-activating factor (PAF) antagonistic activities in addition to inhibitory effects on microsomal monooxygenase in insects. [11] [12] We focused our attention on developing a practical method for the synthesis of (±)-oxo-parabenzlactone via the route involving the thiyl radical addition-cyclization of hydroximates and for the evaluation of pharmacological activities of the lactones prepared.

Thiyl radical addition-cyclization of the readily available allyl hydroximate 16 [5] in the presence of thiophenol (1 equiv.) and AIBN (0.5 equiv.) proceeded smoothly to give ca. 2 : 1 mixture of the cyclized products 17 and 18 in 73% combined yield, which was separated. The unstable cis-17 was readily isomerized into the trans-isomer 18 upon treatment with sodium ethoxide in ethanol. [13]
Hydrolytic conversion of the cyclic hydroximate 18 into the lactone 19 was readily achieved in 96% yield by treatment with 10% HCl in methanol.
Introduction of the benzyl alcohol moiety into the 4-position of the g-lactone ring was readily achieved according to the conventional route involving Pummerer rearrangement and the subsequent Grignard reaction. Oxidation of the trans-sulfide 19 with m-chloroperbenzoic acid (mCPBA) at 0 °C gave the corresponding sulfoxide 20 in 81% yield while oxidative conversion of the cyclic hydroximate 18 to the desired sulfinyl lactone 20 proceeded ineffectively to give the lactone 20 in only 19% yield under the condition using [hydroxy(tosyloxy)iodo]benzene as an oxidant. The trans-sulfoxide 20 was subjected to the Pummerer reaction and the subsequent hydrolysis to afford the desired trans-aldehyde 21 in 84% yield.
Treatment of the aldehyde 21 with the Grignard reagent 22 gave a diastereomeric mixture of the adducts 23, which without separation was converted into the ketone 15 (mp. 138-139 °C (lit. [8] (+)-15: 105-106 °C) by oxidation with pyridinium chlorochromate (PCC) in 45% yield. The ketone 15 obtained was identical with oxo-parabenzlactone[8] upon comparisons of the spectral data with those of the authentic sample.