Synthesis of 3,5-disubstituted 2-aminopyrazines by Pd-mediated
cross-couplings and its use for preparing chemi- and/or bioluminescent
compounds.
Hideshi Nakamura, Daisuke Takeuchi, Mihoko Aizawa, Chun Wu and Akio
Murai
Division of Chemistry, Graduate School of Science, Hokkaido University,
Sapporo 060
e-mail addresshnak@hucc.hokudai.ac.jp
Introduction
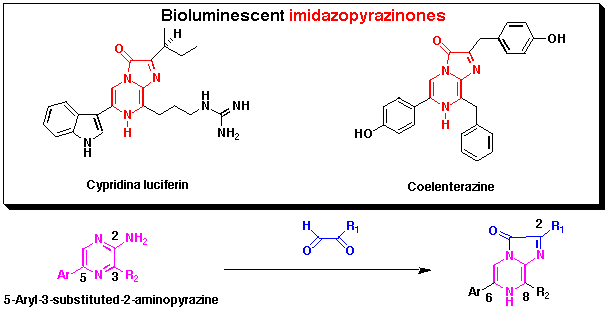
3,6,8-Trisubstituted imidazopyrazine-2-ones are widely distributed in marine organisms
and in some cases are used as bioluminescent substances such as a jellyfish Aequorea aequorea
and a crustacea Cypridina (Vargula) hirgendorfii. These compounds are also chemiluminescent
and utilized to detect chemicals such as Ca2+ or active
oxygen species such as superoxide. We have investigated convergent synthesis
in order to prepare imidazopyrazinones with different luminescent properties such as color of luminescence.
Since 5-aryl-3-substituted-2-aminopyrazines have been used to prepare chemi- and/or
bioluminescent imidazopyrazinones, we have developed a new approach to synthesize
3,5-disubstituted 2-aminopyrazines having conjugated chromophores from commercially
available 2-aminopyrazine by several steps including palladium-mediated cross-coupling as a key reaction.
Results and discussion
I. Synthesis of 3,5-disubstituted 2-aminopyrazines from 2-aminopyrazine.
II. Synthesis of chemi- and/or bioluminescent imidazopyrazinones.
Conclusion
2-Aminopyrazine is shown to be an useful starting material to prepare various types of chemi- and bioluminescent imidazopyrazinones which allow to control the luminescet properties such as the color of luminescence and the rate determining steps. The method described above may allow to supply bioluminescent analogues by short step synthesis, which could be use to investigate the detailed mechanisms of bioluminescence. Bioluminescent compounds together with bioluminescent enzymes will provide a new tool for studies on time-dependent biological phenomena.

