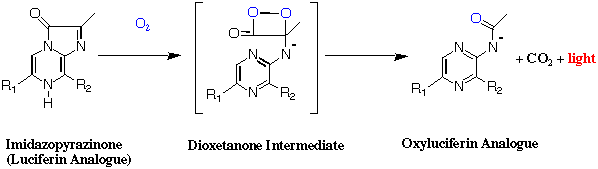

Imidazopyrazinones are well known to react with a molecular oxygen under certain conditions such as in polar aprotic solvents with a catalyst of base or buffer solutions. The oxidation yields the corresponding amide through the dioxetanone intermediate and results in light emission from a excited state of the aminde anion. Imidazopyrazinones with different substituents at the 6 and 8 positions were prepared from the synthesized 2-aminopyrazines with methylglyoxal and their chemiluminescence examined.
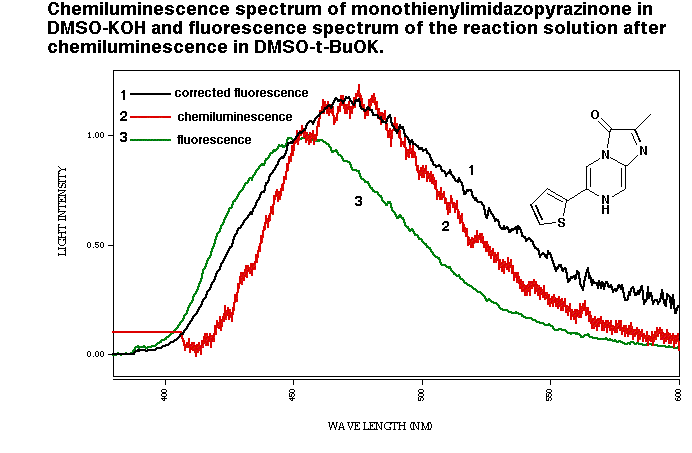
All of the synthesized compounds gave luminescence in Me2SO and most of their chemiluminesce spectrum was identical to the fluorescence spectrum of the amide anions. For example, monothienyl derivative gave blue luminescence with a maximum at 475 nm in Me2SOin the presence of either pH 5.6, 0.1 M acetate buffer or 1M KOH. The chemiluminescence spectrum was superimposable on the corrected fluorescence spectrum of the reaction solution after the emission was triggered by ButOK.
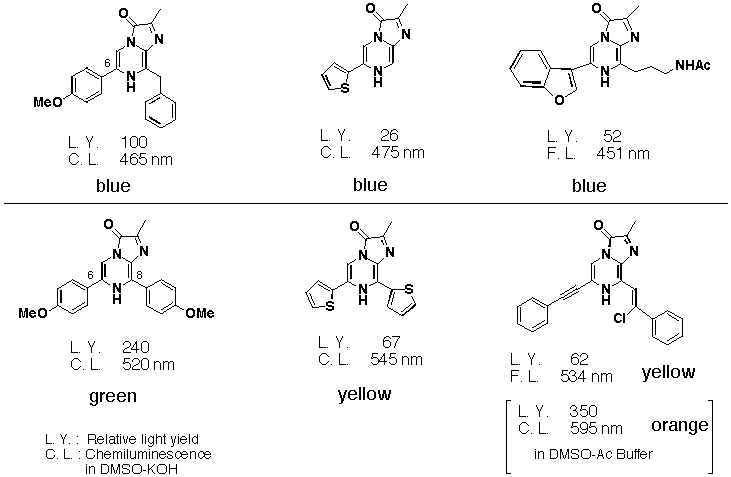
Conjugation at the 6 position of imidazopyrazinone had little effect on luminescence colour whereas conjugation at the 8 position gave a larger shift in luminescence. Coplanarity of the thienyl group resulted in a larger shift than p-MeOPh. The alkynyl compound gave an orange colour in Me2SO-Ac buffer (the longest wavelength so far) which was not identical to the fluorescence spectrum of the reaction mixture under either neutral or strongly basic conditions. Identification of the reaction products and the light emitting species are now in progress. Alkynyl substituents at the 6 position changed pH dependency of the reaction rate of chemiluminesce. The reaction rate of the alkynyl derivatives under basic conditions was slower than that under acidic conditions, whereas basic conditions enhanced the luminescence rate of the other imidazopyrazinones.
 Home
Home