| [Molecules: 33] [Related articles/posters: 024 052 104 018 039 ] |

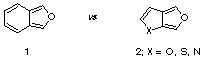
Over the years, most of the studies reported in the literature for these systems centre on their use as dienes in inter- and intra-molecular Diels-Alder reactions [3-10]. Such cycloaddition processes allow for a rapid entry into complex polyheterocyclic rings and makes these compounds potentially useful for natural product synthesis.
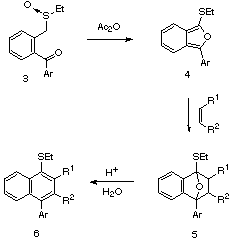
The vast majority of 2,3-methylene heteroaromatics have been prepared by flash vacuum pyrolysis or by 1,4-elimination from suitable precursors. One limitation of these methods is that the precursors are sometimes not easily available. Recently, we reported on the Pummerer-induced cyclization of keto sulfoxides [11] as a method to prepare thio-substituted isobenzofurans of type 4. The a-thio-carbocation generated from the Pummerer reaction of an o-benzoyl substituted sulfoxide is intercepted by the adjacent keto group to produce isobenzofuran 4 as a transient intermediate which undergoes a subsequent Diels-Alder cycloaddition with an added dienophile. The resulting cycloadduct 5 can be readily converted to representatives of several types of aryl naphthalene lignans [12]. In a continuation of our studies in this general area, we now report on an extension of the tandem Pummerer Diels-Alder sequence for the synthesis of several thieno[2,3-c]furans and furo[3,4-b]indoles. The results of these studies are described herein.
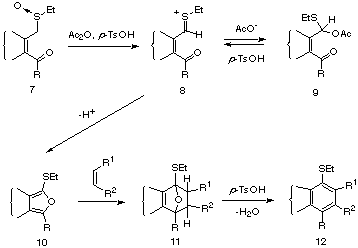
The presence of p-TsOH as a co-catalyst dramatically accelerated the rate at which sulfoxides of type 7 undergo the Pummerer transformation (7 -> 8) as compared to reactions carried out without any p-TsOH. The initially formed thionium ion 8 can either be captured internally by the adjacent carbonyl group [15] to give, after proton loss, the isobenzofuran intermediate 10, or it can react in the traditional sense with an external nucleophile (i.e. acetoxy) to furnish the acetoxy sulfide 9. The presence of p-TsOH effectively drives the reaction in the desired direction (8 -> 10) either by preventing the formation of the acetoxy sulfide 9, or by assisting the ejection of the acetoxy group (9 -> 8), should 9 be formed. The presence of p-TsOH also promotes the conversion of the primary cycloadduct 11 into the aromatized product 12, thereby adding another step to this series of cascade reactions.
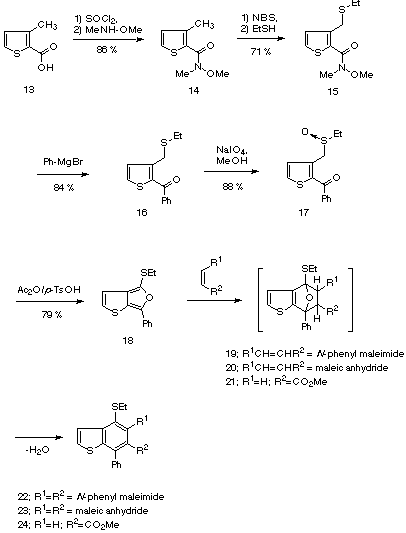
In order to prepare heterocyclic analogues of our previously described a-thioisobenzofurans we first turned our attention to thiophene analogues. Thiophene-2-carboxylic acid 13 was converted to the Weinreb amide 14 following standard procedures [16]. The ethylthiomethyl functionality was then introduced by NBS bromination and subsequent displacement of bromide with ethanethiol [17]. The resulting sulfide 15 was treated with phenylmagnesium bromide to produce ketone 16, which in turn, was oxidized with sodium periodate [18] to give sulfoxide 17. Treatment of 17 with N-phenyl maleimide or maleic anhydride under the tandem Pummerer Diels-Alder conditions provided benzo[b]thiophene derivatives 22 and 23 in excellent yield. The initially formed primary cycloadducts 19 and 20 could not be isolated or observed. However, when the reaction was carried out in the absence of a dienophile, we were able to isolate thieno[2,3-c]furan 18 in 79% yield. This isobenzofuran analogue proved to be surprisingly stable, and to the best of our knowledge corresponds to the second example of a stable thieno[2,3-c]furan reported in the literature [4]. As expected, 18 cleanly afforded thienoisoindole 22 (80%) when treated with N-phenyl maleimide in toluene in the presence of catalytic amounts of p-TsOH at room temperature. It should be noted that thienofuran 18 also underwent the Diels-Alder reaction when the less reactive methyl acrylate was used as the dienophile. Thus, treatment of 18 with methyl acrylate in the presence of scandium(III)trifluoromethanesulfonate [19] for 2 d gave rise to benzo[b]thiophene 24 in 78% yield.
Since furo[3,4-b]indoles have recently been employed as intermediates in the synthesis of some naturally occurring carbazole derivatives (e.g. ellipticine [7], and murrayaquinone A [8]), we decided to explore the potential of the tandem Pummerer Diels-Alder strategy toward the synthesis of several substituted carbazoles. The required sulfoxide 28 was prepared in the standard manner as outlined below. The readily available N-phenylsulfonyl-indole-2-carbaldehyde 25 [20] was first protected as an imine by treating it with tert-butylamine. The resulting imine was subsequently brominated with NBS to give bromomethyl derivative 26. Nucleophilic displacement of the bromide by ethanethiol followed by acidic hydrolysis of the imine produced aldehyde 27. Oxidation of the sulfide in the usual manner gave sulfoxide 28 in 58% overall yield (five steps).
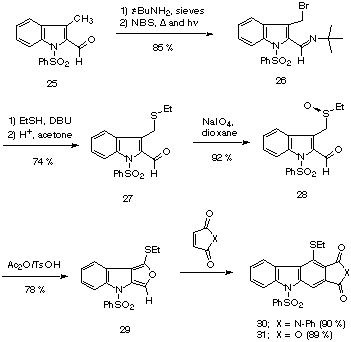
Treatment of 28 with either N-phenyl maleimide or maleic anhydride utilizing the Pummerer Diels-Alder conditions afforded the fused carbazoles 30 and 31 in excellent yield. When the reaction was carried out in the absence of a dienophile, it was possible to isolate furo[3,4-b]indole 29 in 78% yield. Although 29 is not as stable as thienofuran 18, it could be obtained in a high state of purity by rapid work-up and chromatographic purification of the reaction mixture. However, if kept at room temperature, significant decomposition of 18 occurred within days. As expected, a pure sample of furoindole 29 readily reacts with N-phenyl maleimide in the presence of p-TsOH to give pyrrolocarbazole 43.
In conclusion, we have demonstrated that the tandem Pummerer Diels-Alder reaction sequence can be used to efficiently synthesize a variety of polyheterocyclic ring systems. The key intermediates in these cascade processes are a-thio-isobenzofurans, which in some cases can be isolated and independently reacted with an appropriate dienophile to give 4+2 cycloadducts. We found it to be most convenient to carry out these reactions in an all-tandem fashion. Our results clearly indicate that the tandem-cascade process is a powerful method for the construction of complex hetero-aromatic o-quinodimethanes. This area of research is currently being pursued in more detail in our laboratories.
References
1. For a review on isobenzofurans, see: Friedrichsen, W. Adv. Heterocycl.
Chem. 1980, 26, 135. Rickborn, B. Adv. Theoret.
Interesting Molecules, Thummel, R. P., Ed.; Vol. 1, JAI Press: Greenwich,
CT, 1989; p 1. Friedrichsen, W. in Houben-Weyl, Methoden der Organischen
Chemie, Kreher, R. Ed.; Vol. E6b, Thieme Verlag: Stuttgart, 1994; p
163-216.
2. Rodrigo, R. Tetrahedron 1988, 44, 2093.
3. Eberbach, W.; Fritz, H.; Laber, N. Angew. Chem., Int. Ed. Engl. 1988, 27, 568. Eberbach, W.; Laber, N.; Bussenius, J.; Fritz, H.; Rihs, G. Chem. Ber. 1993, 126, 975.
4. Schönig, A.; Debaerdemaeker, T.; Zander, M.; Friedrichsen, W. Chem. Ber. 1989, 122, 1119. Schönig, A.; Friedrichsen, W. Liebigs Ann. Chem. 1989, 405. Schönig, A.; Friedrichsen, W.; Tetrahedron Lett. 1988, 29, 1137.
5. Kuroda, T.; Takahashi, M.; Ogiku, T.; Ohmizu, H.; Nishitani, T.; Kondo, K.; Iwasaki, T. J. Org. Chem. 1994, 59, 7353.
6. Aßmann, L.; Palm, L.; Zander, M.; Friedrichsen, W. Chem. Ber. 1991, 124, 2481. Abszlig;mann, L.; Friedrichsen, W. Heterocycles 1989, 29, 1003. Aßmann, Debaerdemaeker, T.; Friedrichsen, W. Tetrahedron Lett. 1991, 32, 1161.
7. Gribble, G. W.; Keavy, D. J.; Davis, D. A.; Saulnier, M. G.; Pelcman, B.; Barden, T. C.; Sibi, M. P.; Olson, E. R.; BelBruno, J. J. J. Org. Chem. 1992, 57, 5878 and references therein.
8. Miki, Y.; Hachiken, H. Synlett 1993, 333.
9. Shiue, J.-S.; Fang, J.-M. J. Chem. Soc., Chem. Commun. 1993, 1277.
10. Nagel, J.; Friedrichsen, W.; Debaerdemaeker, T. Z. Naturforsch. 1993, B48, 213.
11. Cochran, J. E.; Padwa, A. Tetrahedron Lett. 1995, 36, 3495.
12. Cochran, J. E.; Padwa, A. J. Org. Chem. 1995, 60, 3938.
13. For a review of the Pummerer reaction and different triggering methods, see: DeLucchi, O.; Miotti, U.; Modena, G. Organic Reactions; Paquette, L. A., ed.; John Wiley: 1991, Chap. 3, pp 157-184.
14. Watanabe, M.; Nakamori, S.; Hasegawa, H.; Shirai, K.; Kumamoto, T. Bull. Chem. Soc. Jpn. 1981, 57, 817.
15. DeGroot, A.; Jansen, B. J. M. J. Org. Chem. 1984, 49, 2034. Jansen, B. J. M.; Bouwman, C. T.; DeGroot, A. Tetrahedron Lett. 1994, 35, 2977. Jommi, G.; Pagliarin, R.; Sisti, M.; Tavecchia, P. Synth. Comm. 1989, 2467.
16. Nahm, S.; Weinreb, S. M. Tetrahedron Lett. 1981, 22, 3815. For a review of the chemistry of Weinreb amides, see: Sibi, M., P. Org. Prep. Proced. Int. 1993, 25, 15.
17. Ono, N.; Miyake, H.; Saito, T.; Kaji, A. Synthesis 1980, 952.17.
18. Leonard, N. J.; Johnson, C. R. J. Org. Chem. 1962, 27, 282.
19. For a review on the use of Sc(OTf)3 as catalyst in Diels Alder reactions, see Kobayashi, S. Synlett 1994, 689.
20. Benzies, D. W. M.; Fresneda, P. M.; Jones, R. A. Synth. Commun. 1986, 16, 1799.
4-Ethylthio-6,8-diphenyl-thieno[2,3-f]isoindole-5,7-dione (22) was prepared from 139 mg (0.5 mmol) sulfoxide 17 and 173 mg (1 mmol) N-phenyl maleimide in 91% yield as pale yellow solid, mp 144-145 deg.C; IR (KBr) 1755, 1710, 1500, 1373, 1118, 759, and 693 cm-1; 1H-NMR (CDCl3) d 1.29 (t, 3H, J = 7.5 Hz), 3.25 (q, 2H, J = 7.5 Hz), 7.34-7.59 (m, 10H), 7.75 (d, 1H, J = 5.0 Hz), and 8.07 (d, 1H, J = 5.0 Hz); 13C-NMR (CDCl3) d 15.2, 30.9, 123.5, 126.0, 126.7, 127.8, 128.3, 128.5, 128.8, 129.0, 130.4, 131.7, 132.3, 134.6, 136.0, 146.3, 147.5, 165.8, and 165.9; Anal. Calcd for C24H17NO2S2: C, 69.37; H, 4.12; N, 3.37. Found: C, 69.25; H, 4.15; N, 3.36.
Methyl 4-ethylthio-7-phenyl-benzo[b]thiophene-6-carboxylate (24). A mixture containing 66 mg (0.25 mmol) of thienofuran 18, 215 mg (2.5 mmol) of methyl acrylate, 12 mg (0.025 mmol) of Sc(OTf)3 and 10 mL of dry THF was stirred under argon at 25 deg.C for 2 d. Evaporation of the solvent under reduced pressure followed by silica gel chromatography gave 64 mg (78%) of cycloadduct 24 as a pale yellow oil; IR (neat) 1718, 1432, 1248, and 1127 cm-1; 1H-NMR (CDCl3) d 1.39 (t, 3H, J = 7.5 Hz), 3.10 (q, 2H, J = 7.5 Hz), 3.63 (s, 3H), 7.39-7.48 (m, 5H), 7.62 (m, 2H), and 7.90 (s, 1H); 13C-NMR (CDCl3) d 14.4, 27.9, 52.0, 123.1, 125.8, 126.2, 128.0, 128.3, 128.4, 130.8, 136.2, 139.6, 141.6, 142.4, and 168.0; m/e 328 (M+ , base), 299, 267, 240, 208, 196, 162, and 112; HRMS (EI) Calcd for C18H16O2S2: 328.0592. Found: 328.0583.
10-Ethylthio-2-phenyl-5-phenylsulfonyl-5H-pyrrolo[3,4-b]carbazole-1,3-dione (30) was obtained from 188 mg (0.5 mmol) sulfoxide 28 and 173 mg (1 mmol) N-phenylmaleimide as colourless solid in 90% yield, mp 249-250 deg.C; IR (KBr) 1760, 1708, 1370, and 1350 cm-1; 1H-NMR (CDCl3) d1.25 (t, 3H J = 7.5 Hz), 3.21 (q, 2H, J = 7.5 Hz), 7.39-7.69 (m, 10H), 7.91 (d, 2H, J = 7.8 Hz), 8.46 (d, 1H, J = 8.4 Hz), 8.92 (s, 1H), and 9.27 (d, 1H, J = 8.1 Hz); 13C-NMR (CDCl3) d 15.0, 31.3, 110.0, 114.6, 124.4, 125.4, 125.7, 126.5, 126.6, 127.2, 128.0, 129.0, 129.3, 129.5, 131.6, 131.7, 131.9, 132.8, 134.5, 137.4, 139.6, 141.1, 165.9, and 166.2; Anal. Cacld for C28H20N2O4S2: C, 65.61; H, 3.93; N, 5.47. Found: C, 65.67; H, 3.96; N, 5.46.