Double asymmetric induction of catalytic hetero-Diels-Alder
reactions of prochiral sulfonyl-substituted a,b-unsaturated ketones with vinyl ethers
Eiji Wada,*a Hideyasu
Kawamoto,b Wen Peib and Shuji Kanemasaa
aInstitute of Advanced Material Study, Kyushu University, Kasugakoen,
Kasuga 816, Japan
bDepertment of Molecular Science and Technology, Interdisciplinary
Graduate School of Enginnering Sciences, kyushuUniversity, Kasugakoen, Kasuga
816, Japan
Recently, we have found that (E)-1-phenylsulfonyl-3-alken-2-ones
as sulfonyl-functionalized chelating enones work effectively as hetero 1,3-dienes
in chiral Lewis acid-catalysed hetero-Diels-Alder reactions with vinyl ethers.
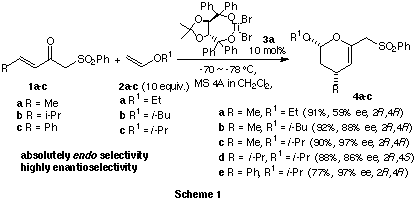
In the presence of a chiral TADDOLTiBr2 catalyst 3a, enones
1a-c were reacted with vinyl ethers 2a-c to produce the corresponding
(2R,4R) or (2R,4S)-cis-2-alkoxy-4-substituted-3,4-dihydro-2H-pyrans
4a-e in absolutely endo- and highly enantioselective manners
(Scheme 1). The enantioselectivity was effectively enhanced with the increase
of bulkiness of the alkoxyl substituent R1 of dienophiles 2a-c (2a<2b<2c).1)
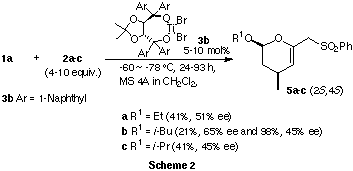
When TADDOLTiBr2 catalyst 3b was used in the reactions of
1a and 2a-c, corresponding cycloadducts with opposite absolute
configuration (2S,4S) were obtained in 45 to 65% ee (Scheme 2). The reaction
with 3b as the catalyst proceeds much slower compared with 3a
as the catalyst.
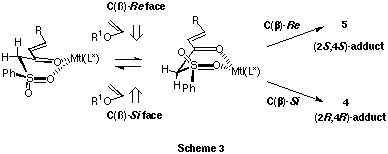
This reaction include the initial step of formation of two diastereomeric
enone/Lewis acid complexes, followed by the second step of four possible
enantioselective ring forming processes (Scheme 3). It is not certain so
far which plays the more important role in the determining step of chiral
induction, the shielding substituents of chiral ligands or the phenyl substituent
on the sulfonyl moiety.
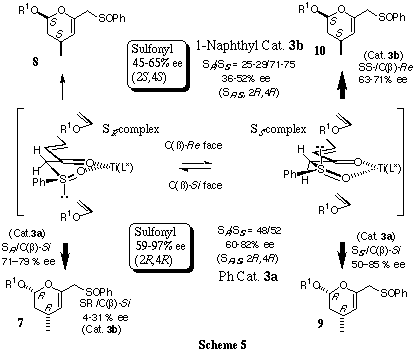
In order to confirm the chirality-determining step among the four possible
approaches, the double chiral induction between rac-(E)-1-phenylsulfinyl-3-penten-2-one
(6) and a chiral titanium catalyst 3 was examined. The reactions of 6
with 2a,c were carried out in the presence of catalyst 3a,b
(50 mol%) to produce possible four diastereomeric endo-cycloadducts
(Scheme 4).
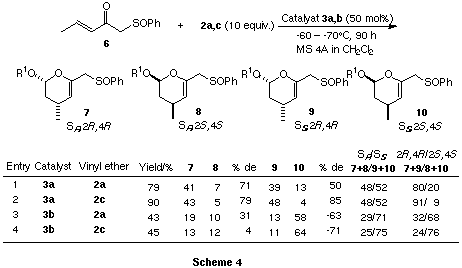
The results are obtained by the use of catalyst 3a as follows:
- The SR and SS complexes have similar reactivity [SR/SS
(7+8/9+10) = 48/52]
- The chirality of the reaction is independent
of the sulfur chirality (7+9>8+10)
- The chirality
of the reaction depends upon the catalyst chirality (7>8
and 9>10)
- Good accordance betweeen sulfinyl- and sulfonyl-functionalized
enone in enantioselectivity (2R,4R).
On the other hand, the
results obtained by the use of catalyst 3b are as follows.
- The SS complex predominantly participates in the reaction [SR/SS
(7+8/9+10) = 29-25/71-79]
- The chirality of the reaction is highly
dependent upon the sulfur chirality [(7>8 (4-31% de) and
10>9 (63-71% de)]
- Good accordance betweeen sulfinyl-
and sulfonyl-functionalized enone in enantioselectivity (2S,4S).
Furthermore, (E)-1-methylsulfonyl-3-penten-2-one (11) was
utilized to evaluate the effect of the sulfonyl substituent on the chirality
induction. The reaction of enone 11 and vinyl ether 2a-c was
carried out in the presence of catalyst 3a,b. The results are summarized
in Scheme 6.
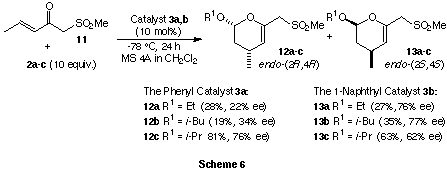
The mode of enantioselectivity is the same to that of enone 1a. Both lower reactivity and
enantioselectivity (22-76% ee) were observed than
for 1a (59-97% ee) with 3a as the catalyst. On the other
hand, slightly higher enantioselectivity was observed than for enone 1a
with 3b as the catalyst. The similar reactivity of 11 regardless
of size of the catalyst 3a,b is in contrast to that of
enone 1a (reactivity: cat. 3a>cat. 3b). These results
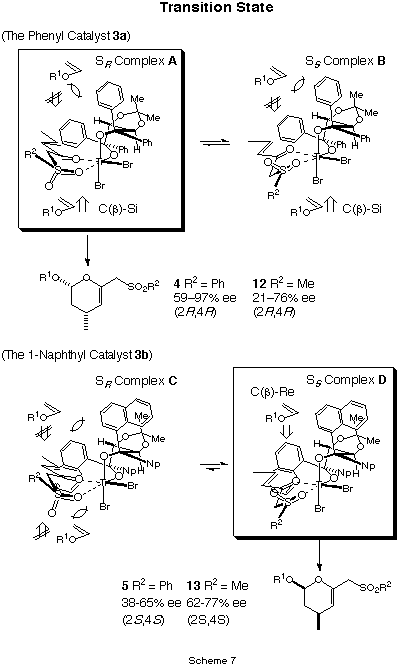
indicate that both the bulkiness of shielding substituents (Ar) of the chiral
ligands and sulufonyl substituents play an important role in the formation
of two diastereomeric enone/Lewis acid complex. Thus, the SR complex
A predominantly participates to provide cycloadduct 4 (2R,4R)
by the reactions of enone 1a and 2 with catalyst 3a.
On the other hand, when catalyst 3b was used in the same reactions, the
SS complex D predominantly participates to provide
cycloadduct 5 (2S,4S) (Scheme 7).
References
- E. Wada, H. Yasuoka, and S. Kanemasa, Chem. Lett., 1994, 1637.






