 ECHET96 Article 007: Gilbert Kirsch
ECHET96 Article 007: Gilbert Kirsch
 ECHET96 Article 007 Nagatoshi Nishiwaki
ECHET96 Article 007 Nagatoshi Nishiwaki
Nitropyrimidinones: synthetic equivalents of unstable nitromalonaldehyde and diformylamine
Department of Chemistry, Osaka Kyoiku University, Asahigaoka 4-698-1, Kashiwara, Osaka 582, Japan
Introduction
Synthetic route to nitropyrimidinones
Reaction of 1-methyl-5-nitropyrimidin-2(1H)-one 4 with
bidentate ions
Ring transformation of 1-methyl-5-nitropyrimidin-2(1H)-one
4
Reaction of 3-methyl-5-nitro-4-pyrimidin-(3H)-one 5 with bidentate ions
Acknowledgements
References
Introduction
The ring transformation of N-substituted
3,5-dinitropyridin-2(1H)-one 1 affords polyfunctionalized
systems such as phenols 3,1 pyridotriazines,1
anilines,2 nitropyridines3 and so on. The stepwise
nucleophilic addition of bidentate anion to pyridone 1 produced the
bicyclic intermediate 2, from which anionic nitroacetamide is eliminated
to give the ring transformed products. In these reactions, pyridone 1
behaves as the synthetic equivalent of unstable nitromalonaldehyde.
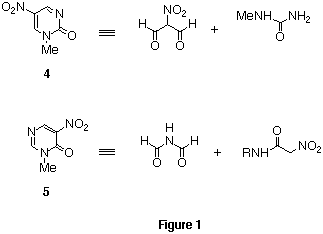
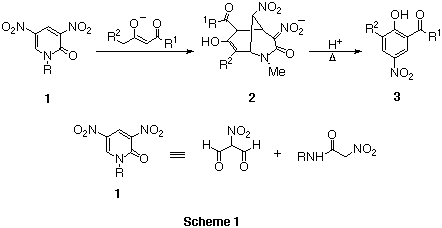 Two isomeric nitropyrimidinones 44 and
55 are azaanalogs of pyridone 1, which have a ring
nitrogen instead of a nitro group. Thus, these compounds are considered to
show similar electron deficiency and reactivities to dinitropyridone 1.
Namely, 1-methyl-5-nitropyrimidin-2(1H)-one 4 consists of the
masked nitromalonaldehyde and N-methylurea, and
3-methyl-5-nitropyrimidin-4(3H)-one 5 consists of activated
diformylamine and N-methylnitroacetamide.
Two isomeric nitropyrimidinones 44 and
55 are azaanalogs of pyridone 1, which have a ring
nitrogen instead of a nitro group. Thus, these compounds are considered to
show similar electron deficiency and reactivities to dinitropyridone 1.
Namely, 1-methyl-5-nitropyrimidin-2(1H)-one 4 consists of the
masked nitromalonaldehyde and N-methylurea, and
3-methyl-5-nitropyrimidin-4(3H)-one 5 consists of activated
diformylamine and N-methylnitroacetamide.
 In this poster, we show the synthetic utilities of two isomeric nitropyrimidinones 4 and 5.
In this poster, we show the synthetic utilities of two isomeric nitropyrimidinones 4 and 5.
Synthetic route to nitropyrimidinones
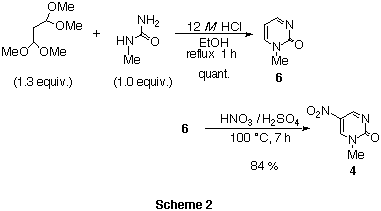
The preparation of both nitropyrimidinones 4 and 5 are easily
achieved in a few steps from commercially available reagents.
1-Methylpyrimidin-2(1H)-one hydrochloride 6 was quantitatively
obtained by the condensation6 of N-methylurea and
1,1,3,3-tetramethoxypropane under acidic conditions. The nitration of
pyrimidinone 6 with
15 M HNO3 in 18 M H2SO4 at 100 celsius
afforded nitropyrimidinone 4 in 84% yield (from urea).
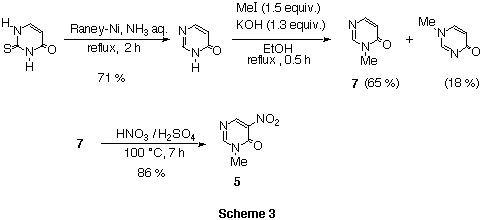
 Nitropyrimidinone 5 was obtained from 2-thiouracil by
reduction,7 methylation,7 and nitration with fuming
HNO3 in 18 M H2SO4 at 100 celsius in 40% overall yield.
Nitropyrimidinone 5 was obtained from 2-thiouracil by
reduction,7 methylation,7 and nitration with fuming
HNO3 in 18 M H2SO4 at 100 celsius in 40% overall yield.
Reaction of 1-methyl-5-nitropyrimidin-2(1H)-one 4 with
bidentate ions
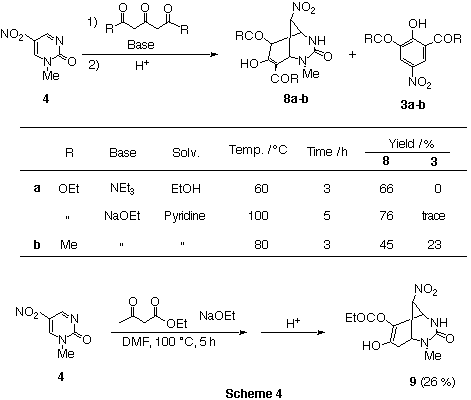
Reaction of nitropyrimidinone 4 with diethyl acetonedicarboxylate in
the presence of NEt3 in EtOH afforded
6,8-bis(ethoxycarbonyl)-7-hydroxy-2-methyl-9-nitro-3-oxo-2,4-diazabicyclo[3.3.1]ona-7-ene
(8a) in 66% yield. In this case, the ring transformed product,
trisubstituted phenol 3,1 was not detected. The same product 8a was also obtained in 76% yield when NaOEt and pyridine were used as the base and the solvent respectively.
However employment of NaOEt was necessary to obtain 9 in the
reaction for ethyl acetoacetate which has only one active methylene group.
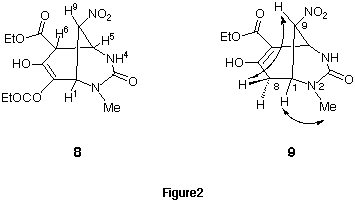
 The structures of bicyclic compounds 8 and 9 were confirmed by
comparison of spectral data with those of 2 being a similar structure.
The positions of the double bond in the ring skeletons is different. The product
8a was bicyclonona-7-ene derivative since spin-spin coupling was
observed between H5 and four protons, H1, H4, H6, and H9, in the 2D
1H-1H COSY spectra. On the other hand, product 9
was bicyclonona-6-ene derivative since NOEs were observed between the proton at
the 1-position and the N-methyl group, and between the proton at
9-position and the exo proton at the 8-position in the 2D
1H-1H NOESY spectra.
The structures of bicyclic compounds 8 and 9 were confirmed by
comparison of spectral data with those of 2 being a similar structure.
The positions of the double bond in the ring skeletons is different. The product
8a was bicyclonona-7-ene derivative since spin-spin coupling was
observed between H5 and four protons, H1, H4, H6, and H9, in the 2D
1H-1H COSY spectra. On the other hand, product 9
was bicyclonona-6-ene derivative since NOEs were observed between the proton at
the 1-position and the N-methyl group, and between the proton at
9-position and the exo proton at the 8-position in the 2D
1H-1H NOESY spectra.
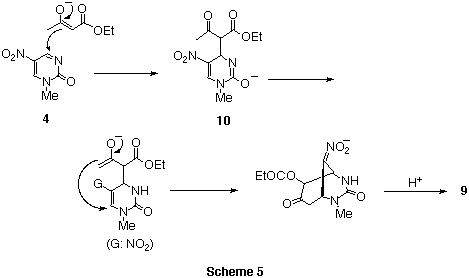
 Based on these facts, the first attack of enolate ions occurred at the less
hindered 4-position of pyrimidinone 4 to furnish the Meisenheimer like
complex 10. The intramolecular addition of this adduct afforded
bicyclic compounds 8 or 9. In the case of diethyl
acetonedicarboxylate, enolization occurred towards the less bulky direction
affording product 8.
Based on these facts, the first attack of enolate ions occurred at the less
hindered 4-position of pyrimidinone 4 to furnish the Meisenheimer like
complex 10. The intramolecular addition of this adduct afforded
bicyclic compounds 8 or 9. In the case of diethyl
acetonedicarboxylate, enolization occurred towards the less bulky direction
affording product 8.
Ring transformation of 1-methyl-5-nitropyrimidin-2(1H)-one
4
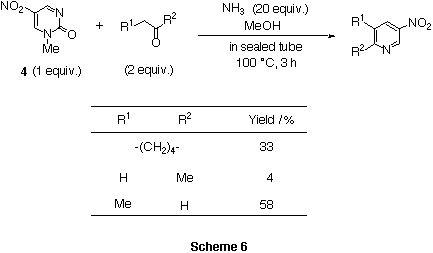
We reported that dinitropyridone 1 reacted with ketones in the presence
of NH3 to give 3-nitropyridine derivatives in good yields.3)
Thus, a similar ring transformation using nitropyrimidinone 4 was examined.
3-Nitropyridine derivatives were obtained but in low yields and the reaction
mixture was complicated.
 Compared with dinitropyridone 1, ring transformation of
nitropyrimidinone 4 barely progressed, and the intermediate bicyclic
compound was more readily isolated. The different
reactivities were caused by a difference in stability of the leaving group, the
anion of urea and nitroacetamide. It was reported that nitropyrimidinone having no substituent at the 1-position also gave a similar product under acidic conditions.4
Compared with dinitropyridone 1, ring transformation of
nitropyrimidinone 4 barely progressed, and the intermediate bicyclic
compound was more readily isolated. The different
reactivities were caused by a difference in stability of the leaving group, the
anion of urea and nitroacetamide. It was reported that nitropyrimidinone having no substituent at the 1-position also gave a similar product under acidic conditions.4
It was found that nitropyrimidinone 4 behaved as the synthetic
equivalent of nitromalonaldehyde, and was an excellent precursor for
polyfunctionalized bicyclic compounds.
Although nitromalonaldehyde is a useful
building block for constructing polyfunctionalized nitro compounds, it is
too unstable to be isolated. As the synthetic equivalent of
nitromalonaldehyde, its sodium salt has been employed for many years. Sodium
nitromalonaldehyde, however, is used restrictively only in aqueous media,
and should be handled as an explosive material before
purification.8 Thus, an improvement in the other facile reagent
substituting for nitromalonaldehyde is an important goal. From
this point of view, nitropyrimidinone 4 and dinitropyridone 1 can
be utilized as the synthetic equivalents of nitromalonaldehyde in organic
media.9
Reaction of 3-methyl-5-nitro-4-pyrimidin-(3H)-one 5 with
bidentate ions
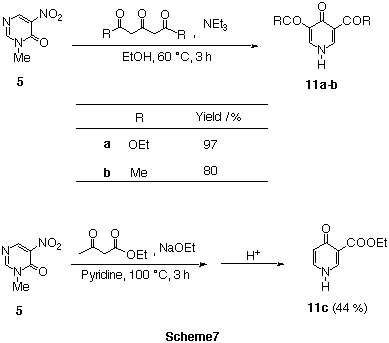
To a solution of nitropyrimidinone 5 in EtOH, diethyl
acetonedicarboxylate and NEt3 were added, and heated. During the
reaction, 3,5-bis(ethoxycarbonyl)pyridin-4(1H)-one 11a
precipitated. The present reaction was applicable to 2,4,6-heptatrione to yield
pyridone derivative 11b. In the case of ethyl acetoacetate, the use
of NaOEt was necessary. In these reactions, nitropyrimidinone
5 behaved as the synthetic equivalent of diformylamine.
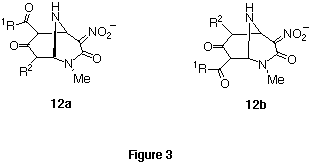
 The present reaction is considered to proceed as follows. Stepwise
nucleophilic addition of bidentate ions to the 2- and 6-positions of
pyrimidinone 5 furnished bicyclic intermediate 12. The anion of
nitroacetamide is eliminated from 12 to give ring transformed products,
3,5-bis(functionalized)pyridones. Although two intermediates 12a and
12b were plausible, it was not determined which compound was produced
since the bicyclic intermediate was not isolated. In this
case, the stable leaving group, the anion of nitroacetamide, facilitated the ring transformation.
The present reaction is considered to proceed as follows. Stepwise
nucleophilic addition of bidentate ions to the 2- and 6-positions of
pyrimidinone 5 furnished bicyclic intermediate 12. The anion of
nitroacetamide is eliminated from 12 to give ring transformed products,
3,5-bis(functionalized)pyridones. Although two intermediates 12a and
12b were plausible, it was not determined which compound was produced
since the bicyclic intermediate was not isolated. In this
case, the stable leaving group, the anion of nitroacetamide, facilitated the ring transformation.
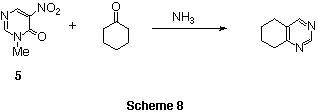
 We have reported that the ring transformation of nitropyrimidinone 5
with ketones in the presence of ammonia readily affords
disubstituted pyrimidine derivatives in which the C-N-C unit is derived from
nitropyrimidinone 5.5
We have reported that the ring transformation of nitropyrimidinone 5
with ketones in the presence of ammonia readily affords
disubstituted pyrimidine derivatives in which the C-N-C unit is derived from
nitropyrimidinone 5.5
 Ring transformations of nitropyrimidines, 5-nitrouracils and
5-nitropyrimidin-2(1H)-ones have been energetically
investigated.10 Whereas there is no instance using
5-nitropyrimidin-4(3H)-ones to the best of our knowledge. Although some
investigation utilizing diformylamine have been reported, few instances applying it
to organic syntheses are known.11 Furthermore, diformylamine
reveals greater amide character than formyl, undergoing nucleophilic
substitution more readily than nucleophilic addition. In the
present reaction, the ring transformed product is formally the latter reaction type,
hence nitropyrimidinone 5 would be a useful synthetic reagent for
constructing the C-N-C unit.
Ring transformations of nitropyrimidines, 5-nitrouracils and
5-nitropyrimidin-2(1H)-ones have been energetically
investigated.10 Whereas there is no instance using
5-nitropyrimidin-4(3H)-ones to the best of our knowledge. Although some
investigation utilizing diformylamine have been reported, few instances applying it
to organic syntheses are known.11 Furthermore, diformylamine
reveals greater amide character than formyl, undergoing nucleophilic
substitution more readily than nucleophilic addition. In the
present reaction, the ring transformed product is formally the latter reaction type,
hence nitropyrimidinone 5 would be a useful synthetic reagent for
constructing the C-N-C unit.
Acknowledgments
The present work was supported by a Grant-in Aid for Scientific Research (No.
04855170, No. 05640605, No. 07855107) from the Ministry of Education, Science
and Culture of Japan, and supported by Daiichi Pharmaceutical Co Ltd Award in
Synthetic Organic Chemistry, Japan.
References
1 Matsumura, E.; Ariga, M.; Tohda, Y. Tetrahedron Lett.,1979, 1393.;
Matsumura, E.; Ariga, M.; Tohda, Y. Bull. Chem. Soc. Jpn., 1979, 52, 2413.
2 Tohda, Y.; Ariga, M.; Kawashima, T.; Matsumura, E. Chem. Lett.,
1983, 715.; Matsumura, E.; Tohda, Y.; Ariga, M. Bull. Chem. Soc. Jpn., 1982, 55, 2174.
3 Tohda, Y.; Eiraku, M.; Nakagawa, T.; Usami, Y.; Ariga, M.; Kawashima, T.; Tani, K.; Watanabe, H.; Mori, Y. Bull. Chem. Soc. Jpn., 1990, 63,
2820.
4 Fox, J. J.; Su, T.-L.; Stempel, L. M.; Watanabe, K. A. J. Org. Chem., 1982, 47, 1081.
5 Nishiwaki, N.; Matsunaga, T.; Tohda, Y.; Ariga, M. Heterocycles,
1994, 38, 249.
6 Fox, J. J.; Praag, D. V. J. Am. Chem. Soc., 1960, 82, 486.
7 Bauer, L; Wright, G. E.; Mikrut, B. A.; Bell, C. L. J. Heterocycl. Chem., 1965, 2, 447.
8 Fanta, P. E.; Stein, R. A. Chem. Rev., 1960, 60, 261.; Fanta, P. E. Org. Synth., Coll. Vol. 4, 844 (1963).
9 Nishiwaki, N.; Tohda, Y.; Ariga, M. Bull. Chem. Soc. Jpn., 1996,
69, in press.; Tohda, Y.; Ariga, M.; Kawashima, T.; Matsumura, E.
Bull. Chem. Soc. Jpn., 1987, 60, 201.
10 Rusinov, V. L.; Chupakhin, O. N.; van der Plas, H. C. Heterocycles,
1995, 40, 441.
11 Kashima, C.; Arao, H.; Hibi, S.; Omote, Y. Tetrahedron Lett., 1989,
30, 1561.; Flitsch, W.; Hampel, K.; Hohenhorst, M. Liebigs Ann. Chem., 1990, 397.










