 The commercially available '1994 Reagent of the Year',
[1] Jacobsen's catalyst (1), converts achiral olefins to chiral epoxides
with enantiomeric excesses regularly better than 90% [2] and sometimes
exceeding 98%.[3] To date, the origin of this dramatic selectivity has not
been explained.
The commercially available '1994 Reagent of the Year',
[1] Jacobsen's catalyst (1), converts achiral olefins to chiral epoxides
with enantiomeric excesses regularly better than 90% [2] and sometimes
exceeding 98%.[3] To date, the origin of this dramatic selectivity has not
been explained.
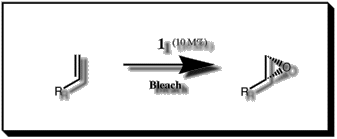 Figure 1 The Jacobsen epoxidation
Figure 1 The Jacobsen epoxidation
Jacobsen's Catalyst (1) is one member of a class of over 75
epoxidation catalysts [4] based on a chiral salen ligand complexed with
Manganese(III) (the base salen ligand in Figure 2 is drawn in red).
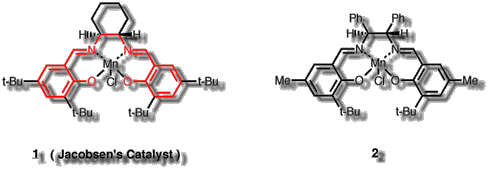 Figure 2 Jacobsen's Catalyst (1) and the first salen catalyst
introduced by Jacobsen (2)
Figure 2 Jacobsen's Catalyst (1) and the first salen catalyst
introduced by Jacobsen (2)
Jacobsen's catalyst is a convenient and effective tool for stereoselective
synthesis as demonstrated by its use in the synthesis of the key side chain
in the antitumour drug Taxol.[5] Jacobsen has recently reported a simple
large-scale preparation of catalyst,[6] making it readily available in
kilogram quantities. The reaction requires only a few percent of catalylst
to substrate while making use of a low cost oxidant - common bleach.[7]
These simple requirements result in less chemical waste, an increasingly
important concern in the chemical industry.[8]
The mechanism of the reaction has two related but distinct aspects: the
mode of oxygen transfer and the method of chiral induction. Neither aspect
is fully understood.
The oxygen transfer is known to occur by a two step catalytic cycle (Figure
3), similar to the accepted mechanism of metal porphyrin catalysed epoxidations.
An oxidant transfers atomic oxygen to the MnIII catalyst in the first
step. The oxygen presumably coordinates to the metal in a site normal to
the porphyrin or salen plane, and the activated oxygen is then delivered
to the alkene. This cycle, as opposed to assisted transfer by a complex
aggregate, is supported by observations that several oxidants have been
used with identical selectivity,[9] that the reaction consumes stoichiometric
quantities of an oxidant in the process of converting an alkene to an epoxide,[9]
and that related salen complexes show incorporation of oxygen from in situ
labelled water,[10] which does not occur via exchange with the oxidant or
epoxide.[11]
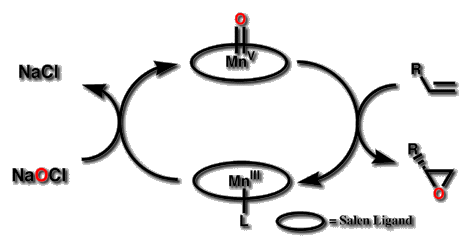
Figure 3 The epoxidation occurs by a two step catalytic cycle
Oxygen transfer from the catalyst to the olefin has been hypothesized to
occur by several different mechanisms (Figure 4) including attack of an
oxygen radical intermediate on the double bond (A), concerted oxygen delivery
(B), and formation of a metallaoxetane intermediate (C).
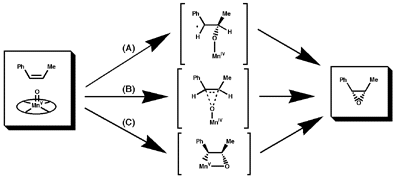 Figure 4 Three possible modes of oxygen delivery
Figure 4 Three possible modes of oxygen delivery
Although Norrby and coworkers have recently proposed a mechanism by which
a metallaoxetane is a plausible intermediate,[12] and Jacobsen has demonstrated
that it is likely oxidation of different olefins occurs by different
mechanisms,[13] it is generally accepted that oxygen transfer to olefins
with radical stabilizing groups occurs by an attack of an oxygen centred
radical on the olefin. This mode of oxygen delivery is supported by the
observation that cis olefins result in both cis and trans epoxides.[14]
The second aspect of the epoxidation mechanism - the cause of stereoselectivity
in the reaction - must occur in the second step of the catalytic cycle
where oxygen is transferred from the oxo-manganese complex to the epoxide.
Fundamental to this stereoselection is the relative orientation of the activated
catalyst and the olefin in the first irreversible bond formation. In the
following section, we will provide a novel hypothesis for the origin of
stereoselection in the Jacobsen Epoxidation.

 Intro | Theory | Calculations | Summary | References
Intro | Theory | Calculations | Summary | References
 The commercially available '1994 Reagent of the Year',
[1] Jacobsen's catalyst (1), converts achiral olefins to chiral epoxides
with enantiomeric excesses regularly better than 90% [2] and sometimes
exceeding 98%.[3] To date, the origin of this dramatic selectivity has not
been explained.
The commercially available '1994 Reagent of the Year',
[1] Jacobsen's catalyst (1), converts achiral olefins to chiral epoxides
with enantiomeric excesses regularly better than 90% [2] and sometimes
exceeding 98%.[3] To date, the origin of this dramatic selectivity has not
been explained.




