My previous three posts set out my take on three principle categories of pericyclic reaction. Here I tell a prequel to the understanding of these reactions. In 1965, Woodward and Hoffmann[1] in their theoretical analysis (submitted Nov 30, 1964) for which the Nobel prize (to Hoffmann only of the pair, Woodward having died) was later awarded. But in the same year, Elias Corey[2] reported the conclusion of a project started several years earlier (first reported (DOI: 10.1021/ja00907a030, Nov 1, 1963) to synthesize the sesquiterpene dihydrocostunolide.
The key element of this synthesis is described as the photochemical ring-opening of 10 to give a thermally unstable ten-membered ring compound 13, which at room temperature recyclizes to 16. These two reactions constitute perfect examples of the Woodward-Hoffmann rules, a modern statement of which is that a 4n+2 electron photochemical pericyclic reaction (for the above example, n=1) normally proceeds with one antarafacial component whilst a thermal one proceeds with only supra facial components (a more recent extension of this statement would be the rule for two antarafacial components). To illustrate this, shown below is the thermal cyclisation of dimethylhexatriene as a model for 13. The IRC shows one interesting conformational feature at ~+8, which is the rotation of the two methyl groups to replace the eclipsed by a gauche orientation. Clearly visible is the suprafacial component (the new bond forms on the bottom face of both termini of the triene) and in the example of 13 → 16 resulting in the observed stereochemistry.
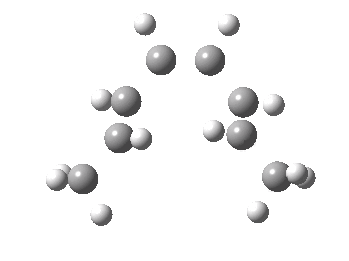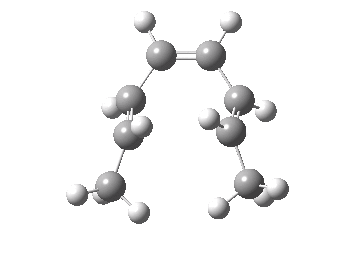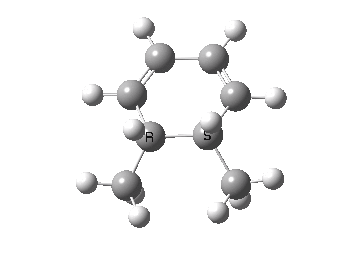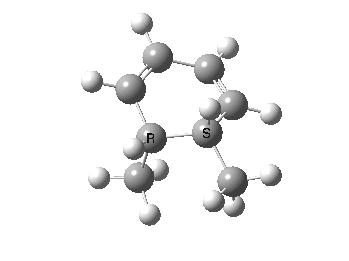 Thermal electrocyclic reaction of a dimethyl-hexatriene. Click for 3D. |
|
The photochemical reaction of 10 can be illustrated by the nature of the conical intersection where the singlet (S0/S1) surfaces touch. The antarafacial component is clearly seen (bottom face of lhs of the triene, top face of the rhs of the other end of the triene), leading to the observed stereochemistry of the photochemical product 13.
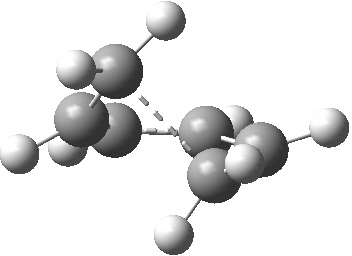
Geometry of a conical intersection for photochemical electrocyclisation of hexatriene. Click for 3D.
So Corey had in his hands in 1963 an unambiguous and clear cut example of stereoselection operating in a pericyclic reaction, and an opportunity in 1965 (if not earlier) to infer and declare a general guiding principle from that reaction. In fact that opportunity was not taken by Corey, and he was (DOI: 10.1021/jo049925d) left to rue decades later on what might have been!
References
- R.B. Woodward, and R. Hoffmann, "Stereochemistry of Electrocyclic Reactions", Journal of the American Chemical Society, vol. 87, pp. 395-397, 1965. https://doi.org/10.1021/ja01080a054
- E.J. Corey, and A.G. Hortmann, "The Total Synthesis of Dihydrocostunolide", Journal of the American Chemical Society, vol. 87, pp. 5736-5742, 1965. https://doi.org/10.1021/ja00952a037
Tags: electrocyclic, Elias Corey, Historical, pericyclic, photochemical, photochemical product, Tutorial material
[…] Henry Rzepa Chemistry with a twist « So near and yet so far. The story of the electrocyclic ring opening of a cyclohexadiene. […]
[…] have written earlier about dihydrocostunolide, and how in 1963 Corey missed spotting the electronic origins of a key step in its synthesis.[1]. A […]