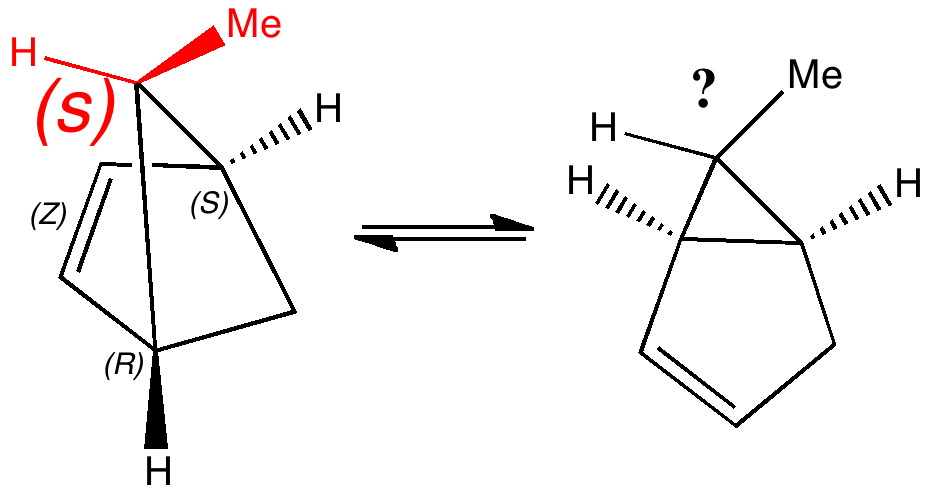
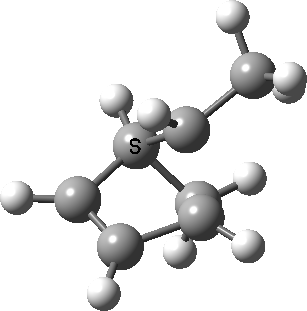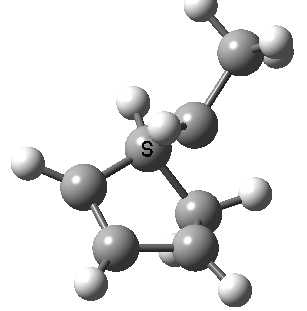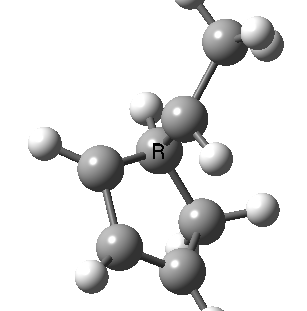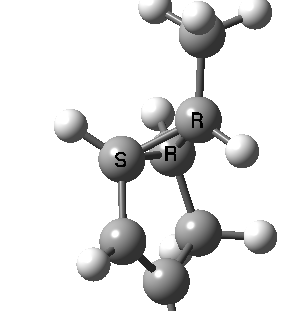
Most representational chemistry generated on a computer requires the viewer to achieve a remarkably subtle transformation in their mind from two to three dimensions (we are not quite yet in the era of the 3D iPad!). The Cahn-Ingold-Prelog convention was a masterwork (which won the Nobel prize). It is shown in action for the molecule on the left below. The CIP notation was actually generated by Chemdraw, and required a fair sprinkling of wedged and hashed bonds to (try to) remove stereoambiguity and generate the labels (try it for yourself). As part of a lecture course on pericyclic reactions, I tell the students that the reaction involves a [1,3] sigmatropic migration of the red carbon and that this migration proceeds with inversion of configuration at this migrating carbon (as the selection rules require). Perceiving what the correct CIP product label should be (with inferred stereochemical labels, resolving ? into either R or S) is IMHO one of the most difficult conceptual experiences in all of organic chemistry. I have over the years struggled to find a way of revealing this in lecture notes (these struggles with the “lecture notes” will be the topic of a future post here). However, I think I may have finally cracked it; my solution is set out below!
A short-cut to the answer might be to reason that if the CIP label for the reactant is (S), and we are told that the carbon migrates with inversion, we should label the product (R). Unfortunately, CIP does not work like that. Because the key carbon in the product is not connected to exactly the same four surrounding ligands as the reactant, all bets are off! Having got that one out of the way, I now uncover my new diagram.
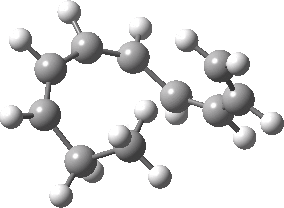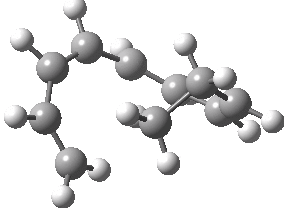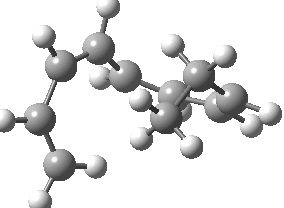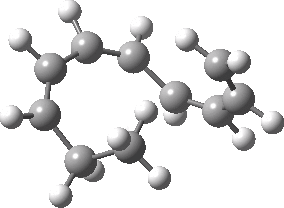
Intrinsic reaction coordinate (IRC) for the 1,3 carbon migration. Click for transition state mode.
It is worth considering how the IRC representation might improve upon the perception compared with the imaginary (transition state) vibrational mode. The latter in effect represents only a small part of that IRC, the so-called harmonic part. Most reactions deviate from such a harmonic oscillator, and the IRC captures that deviation. The stereochemical perception can be enhanced by showing the full (anharmonic) motion of the atoms during the reaction.
Finally, a bonus. Below you can see a [1,9] sigmatropic methyl migration. It proceeds both with inversion at the methyl, and from top to bottom faces (antarafacially) of the nonatetraene. A double-twist Möbius transition state.
Tags: Cahn-Ingold-Prelog, CIP, iPad, pericyclic, sigmatropic, Tutorial material