The Pirkle reagent is a 9-anthranyl derivative (X=OH, Y=CF3). The previous post on the topic had highlighted DIST1, the separation of the two hydrogen atoms shown below. The next question to ask is how general this feature is. Here we take a look at the distribution of lengths found in the Cambridge data base, and focus on another interesting example.
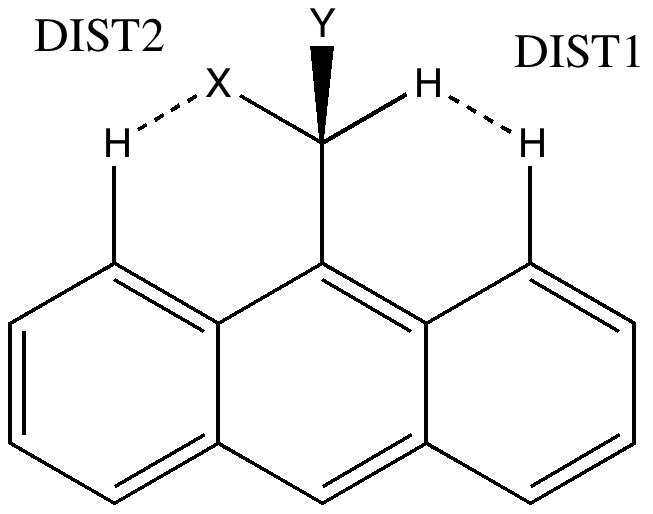
9-anthranyl derivatives. Click for Pirkle with normalised C-H lengths.
The histogram below shows all 9-anthranyl compounds in the CCDC database distributed by DIST1. The search was conducted with the restrictions of no disorder, no “errors”, and using normalised hydrogen bond lengths. A note of explanation for the latter. Because of the nature of x-ray diffraction, when a C-H distance is obtained from a structural refinement, it tends to emerge ~0.1Å to short. Normalisation means adjusting that distance to a more correct 1.09Å (the heavy atom stays put, its the H that moves). In our case, this has the effect of actually shortening DIST1. In the Pirkle structure (shortcode SOCLIF) the nominal value for DIST1 is 1.94-1.96Å, but normalisation reduces these to 1.82-1.85Å. This really is an unusually short contact between two hydrogen atoms (the sum of the vdW radii is 2.4Å). So how unusual might this be? Show below is the result of the CCDC search.
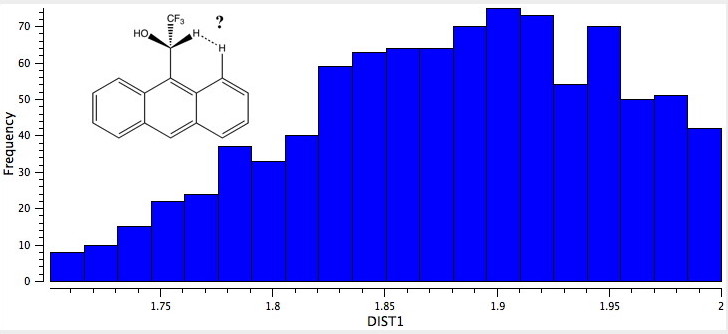
H...H Contacts in 9-anthranyl derivatives
Notice how a maximum in the number of examples is visible at ~1.9Å, but examples all the way down to ~1.7Å are known! If one restricts the search to examples where X=O, the following plot is obtained. The entry on the bottom left is JARYEG, where Y is sufficiently large to enforce short H…H or O…H contacts on both sides. Click on the histogram picture below to see it. When you do so, you will also see the NCI surface computed at this geometry. Note that both the short H..H (DIST1) and the short O…H (DIST2) interaction surfaces are coloured blue, indicating attractive contacts!
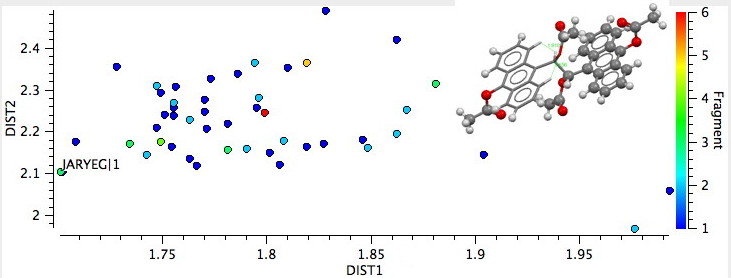
9-anthranyl derivatives, X=O.
If you explore the 3D model further, you will notice other blue interaction surfaces, and a number which have both blue AND orange (= repulsive) zones. We see here yet another example of a weak interaction being simultaneously both attractive and repulsive. It is no longer sufficient to say that the interaction between two atoms is either one or the other. Depending on where you measure it, it can be both! In other words, even weak bonds can have internal structure (for a discussion of the internal structure of a strong C-S bond, see DOI
10.1021/ct100470g).
-
Henry Rzepa is Emeritus Professor of Computational Chemistry at Imperial College London.
View all posts
Related
Tags: 9-anthranyl, Cambridge, disorder, Julia Contreras-Garcia, X-ray
This entry was posted on Tuesday, May 31st, 2011 at 6:51 am and is filed under Interesting chemistry. You can follow any responses to this entry through the RSS 2.0 feed.
You can leave a response, or trackback from your own site.



[…] have done so below using the NCI (non-covalent interaction) procedure (which I have commented on in many other posts here). The NCI surface is shown below, embedded within the NCI surface as purple dots are the BCP […]