Nature has produced most natural molecules as chiral objects, which means the molecule can come in two enantiomeric forms, each being the mirror image of the other. When a natural product is synthesised in a laboratory, a chiral synthesis means just one form is made, and then is compared with the natural product to see if it matches. Just such a process was following in the recent synthesis of cylindricine, a marine alkaloid[1] featured on the ACS molecule-of-the-week site. The authors noted that the absolute configuration of cylindricine as isolated naturally had remained unassigned, and as it happens one way of measuring the properties of the individual enantiomer – its optical rotation – had not been determined. So in part, the purpose of this synthesis was to determine the absolute configuration of this molecule. Here I explore this process.
There are several different procedures for finding the absolute configuration of a molecule.
- By synthesis from a starting material, itself presumed of known absolute configuration – in this example, a molecule[2] which had been previously assigned an absolute configuration. The presumption then is that all the transformations made to this molecule have stereochemically certain and predictable outcomes and of course that the configuration of the starting material in this process was not in any doubt. Ultimately, this chain of inferences should be traceable all the way back to D-(+)-glyceraldehyde. These inference chains can involve multiple groups working at different times.
- Alternative methods can be used as an independent check on the first method above, which depend only on the properties of the target molecule itself and not on any inference chain. One such is the “gold standard”, introduced in 1951[3] and using X-ray crystallography. This method is quite common nowadays, but it does require a suitable crystal for measurement.
- The so-called chiroptical properties of the target molecule can also be subjected to computational prediction, a method first introduced in 1937[4] using the optical rotation as the measure and based on linearly polarized light at a specific wavelength (normally corresponding ot 589nm). As was found in 1937, this can be quite a fragile method, depending very much on the actual conformations of the molecule. Rigid molecules are more predictable than flexible ones. Cylindricine itself has a number of conformations or orientations of the various substituents and it then becomes an question of finding the most stable of these, in terms of their overall contributing populations.
- A more recent method is the use of a technique known Electronic circular dichroism (ECD), which uses circularly rather than linearly polarised light, across a range of wavelengths from ~200 up to ~800nm.
- An even more recent chiroptical method is VCD or Vibrational Circular Dichroism. This spectroscopic technique detects differences in attenuation of left and right circularly polarized light passing through a sample. It is an extension of circular dichroism spectroscopy into the infrared ranges.
Any or all of methods 3-5 could be used to independently check on the results inferred in procedure 1. Here I report the results of such an attempted verification.
The start point is an attempt to find the most stable conformation of cylindricine. Here I am using a conformational tool called GMMX, part of the Gaussview suite. Loading the molecule as drawn above, six rotatable bonds are automatically identified and the program systematically rotates about all of these in turn using a molecular mechanics force field to compute an energy. This field includes so-called dispersion or van der Waals attractions. I used the MMFF94 force field, with its origins in the pharmaceutical industries and reasonably suitable for a natural product. The lowest energy conformation obtained is shown below, but it should be noted that there are 36 further conformations within 3.5 kcal/mol of the lowest. This conformer was chosen for the chiroptical calculations described in 3-5 above. Of course, more thoroughly all the conformers with a population of at least 1% should be included in this process for a more comprehensive analysis.
To get an inkling of why this conformer might be the lowest in energy, inspect the model below (click on the image to get a 3D rotatable model). It shows the so-called NCI (non-covalent-interactions), which are mostly composed of hydrogen bond and dispersion stabilisations. Each little blue/green isosurface is one of these – and the more of them there are – the more stable the conformer.
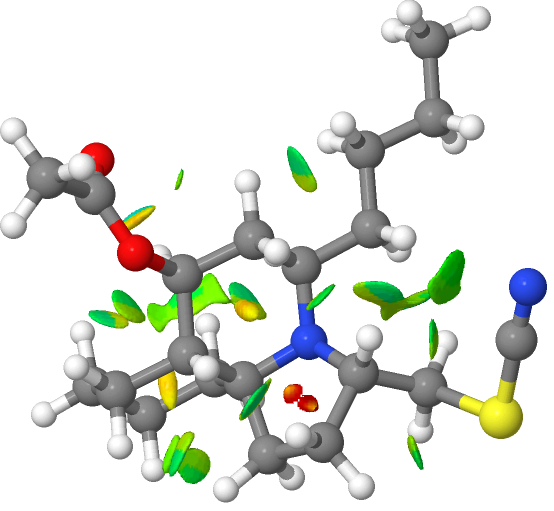
For this conformer, the calculated optical rotation emerge as -34° at 589nm (FAIR Data DOI: 10.14469/hpc/12231). The reported value is -8.5°. You might think that the agreement is poor, but such calculations are only reasonably clear-cut for large values of the rotations! Clearly, this calculation provides some supporting evidence that the assignment of absolute configuration is correct. The take home message is not the value of the rotation but its sign, where calculation and measurement agree. The next step would be to perform a full conformational average over all 37 conformations!
The calculated ECD spectrum is shown below. It only shows a weak negative feature at ~220nm and strong evidence requires features at >280nm to be clear cut. This result suggests that recording this spectrum is not recommended.
The VCD spectrum is shown below. This does show strong features in both the C-H stretching region and the 1500-800 wavenumber region and would be a good diagnostic. Recording it would indirectly also reveal whether the conformer chosen above is likely to be correct or not.
So the above provides a start point for a more comprehensive and independent method for verifying the absolute configuration. The total synthesis using a starting material of known configuration it has to be said is normally pretty reliable, but there are rare examples where a mistake in assignment was made of such a precursor and which was indeed corrected by VCD assignment.[5]
This blog has DOI: 10.14469/hpc/12233
References
- M. Piccichè, A. Pinto, R. Griera, J. Bosch, and M. Amat, "Total Synthesis of (−)-Cylindricine H", Organic Letters, vol. 24, pp. 5356-5360, 2022. https://doi.org/10.1021/acs.orglett.2c02004
- M. Amat, O. Bassas, N. Llor, M. Cantó, M. Pérez, E. Molins, and J. Bosch, "Dynamic Kinetic Resolution and Desymmetrization Processes: A Straightforward Methodology for the Enantioselective Synthesis of Piperidines", Chemistry – A European Journal, vol. 12, pp. 7872-7881, 2006. https://doi.org/10.1002/chem.200600420
- J.M. BIJVOET, A.F. PEERDEMAN, and A.J. van BOMMEL, "Determination of the Absolute Configuration of Optically Active Compounds by Means of X-Rays", Nature, vol. 168, pp. 271-272, 1951. https://doi.org/10.1038/168271a0
- J.G. Kirkwood, "On the Theory of Optical Rotatory Power", The Journal of Chemical Physics, vol. 5, pp. 479-491, 1937. https://doi.org/10.1063/1.1750060
- J.L. Arbour, H.S. Rzepa, A.J.P. White, and K.K.(. Hii, "Unusual regiodivergence in metal-catalysed intramolecular cyclisation of γ-allenols", Chem. Commun., pp. 7125-7127, 2009. https://doi.org/10.1039/b913295c