Previously, I looked at autocatalytic mechanisms where the carboxyl group of an oxetane-carboxylic acid could catalyse its transformation to a lactone, finding that a chain of two such groups were required to achieve the result. Here I look at an alternative mode where the oxetane-carboxylate itself acts as the transfer chain, via a H-bonded dimer shown below.
The IRC energy profile is shown below for a C2-symmetric stationary point in which each molecule catalyses the opening of the other in a concerted manner. The apparent free energy barrier is 72.6 kcal/mol (ωB97XD/Def2-SVPP).
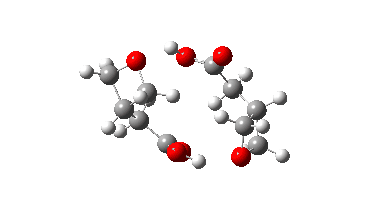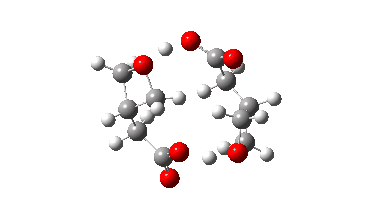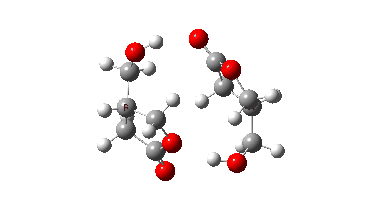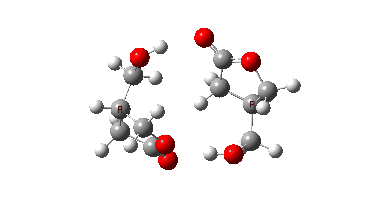
In fact this is what is called a second order saddle point, having two negative force constants in its calculated diagonalised force constant matrix. To remove the unwanted one, it is necessary to find transition states that accomplish the transfer consecutively rather than concurrently. There are two and their IRC energy profiles are stitched together below. This shows also that the proton transfers (IRC -1, +2) also happen asynchronously.
This results in a lower barrier (49.0 kcal/mol), but still far higher than the one obtained using a chain of two carboxylic acids. So this particular version of an autocatalytic transform is not in the event viable.