Having established a viable model for the unexpected isomerism of oxetane carboxylic acids to lactones[1], and taken a look at a variation in the proton transfer catalyst needed to accomplish the transformation, I now investigate the substrate itself.
R’ is set to have three values, R’=H (the original substituent), R’= CH3 and R’= CF3 (FAIR data DOI: 10.14469/hpc/10820)
| R’ | ΔG‡, kcal/mol |
|---|---|
| H | 27.0 |
| CH3 | 29.1 |
| CF3 | 39.6 |
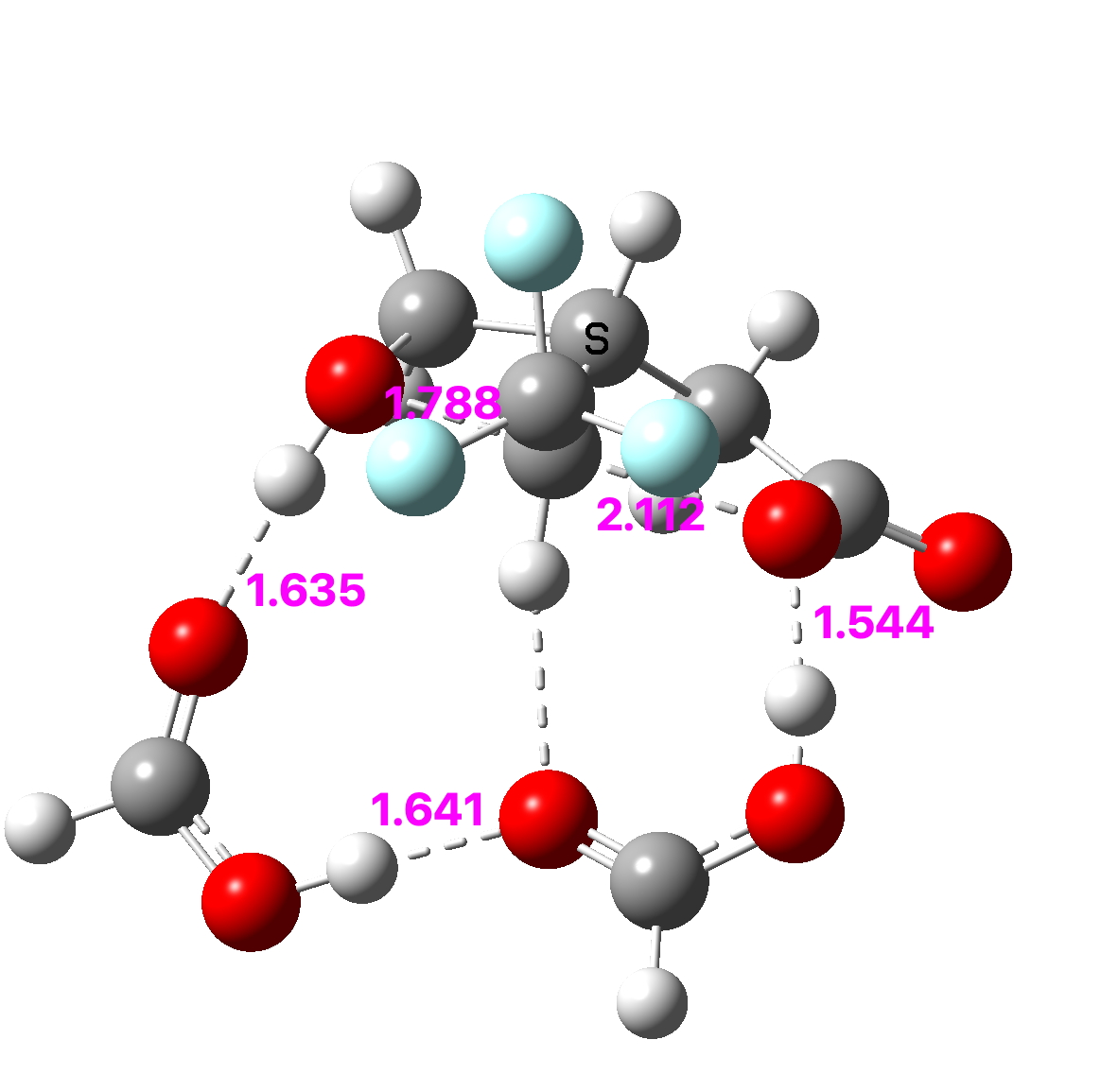
The inference is clear-cut; to inhibit the isomerisation to a lactone, CF3 groups substituted onto the methylene groups of the oxetane will effectively do this, with CH3 itself having a much weaker effect.
DOI: 10.14469/hpc/10861 and 10.14469/hpc/10862
References
- B. Chalyk, A. Grynyova, K. Filimonova, T.V. Rudenko, D. Dibchak, and P.K. Mykhailiuk, "Unexpected Isomerization of Oxetane-Carboxylic Acids", Organic Letters, vol. 24, pp. 4722-4728, 2022. https://doi.org/10.1021/acs.orglett.2c01402
I have also now added a Me at the bridgehead position. The free energy barrier was 29.2 kcal/, pretty much the same as adding the methyl to the methylene gorups.