In the previous post, I showed some of the diverse “non-classical”interactions between Biotin and a protein where it binds very strongly. Here I take a look at two of these interactions to discover how common they are in small molecule structures.
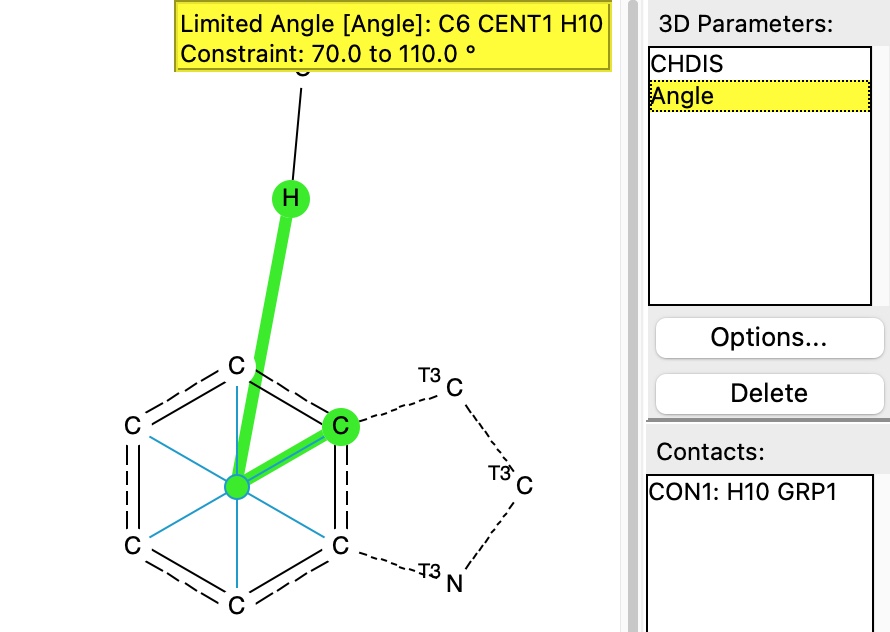
The first search is of a CH hydrogen bond to the face of the aromatic ring in a tryptophane residue
The search is shown below, in which the distance of the hydrogen to the ring centroid is defined, as is the angle subtended at that centroid, constrained to lie within 20° of a vertical approach.
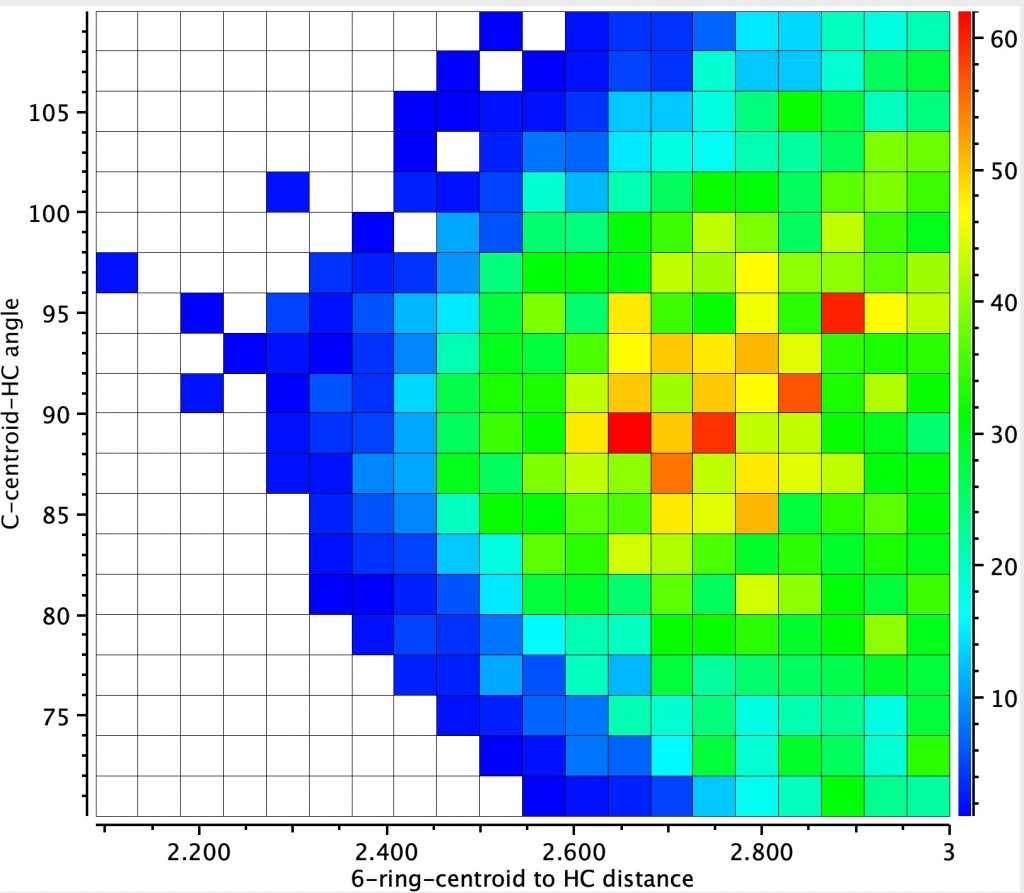
The resulting heat plot shows 2772 entries (no disorder, no errors, R < 0.05), with a rather diffuse red spot at around 2.7-2.8Å (but which can be as short as 2.3Å) and an angle of approach of ~90±5°. This matches the concept of a region of interaction rather than a more focused “hydrogen bond”. It is seen as a relatively common motif!
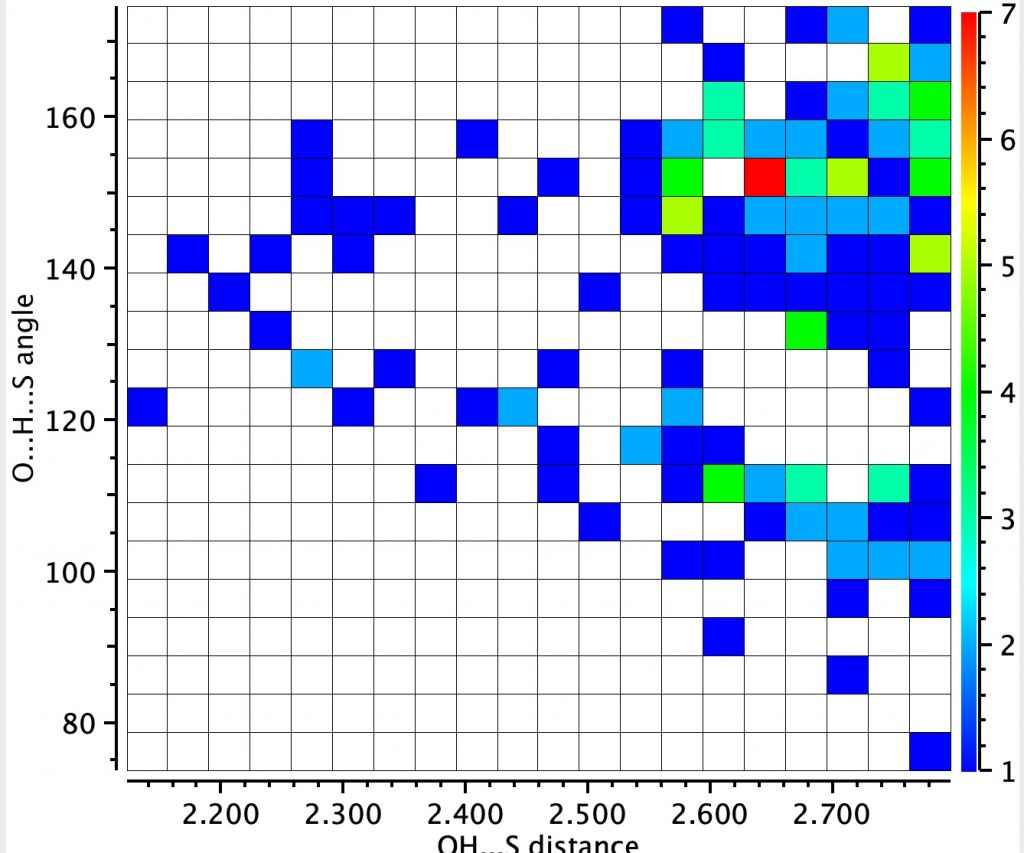
The next search is for “hydrogen bonding” between the sulfur of an C-S-C unit (as found in Biotin) and an OH group. 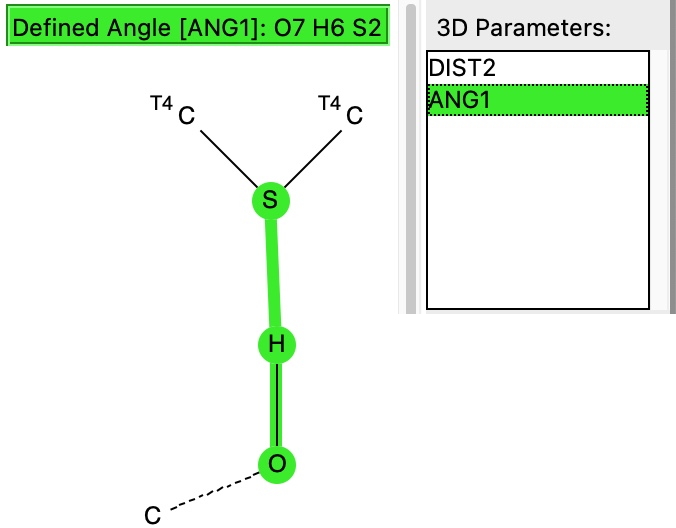
This is less common, with 151 entries in the Cambridge small molecule database, the red spot having a relatively short S…H distance of 1.65Å and a slightly non linear angle.
The NH analogue of this search is shown below (422 hits) shows two clusters. The one with a large angle at H is more concentrated and reveals a distance of ~2.9Å whilst the second cluster has smaller angle and a long tail out to ~2.5Å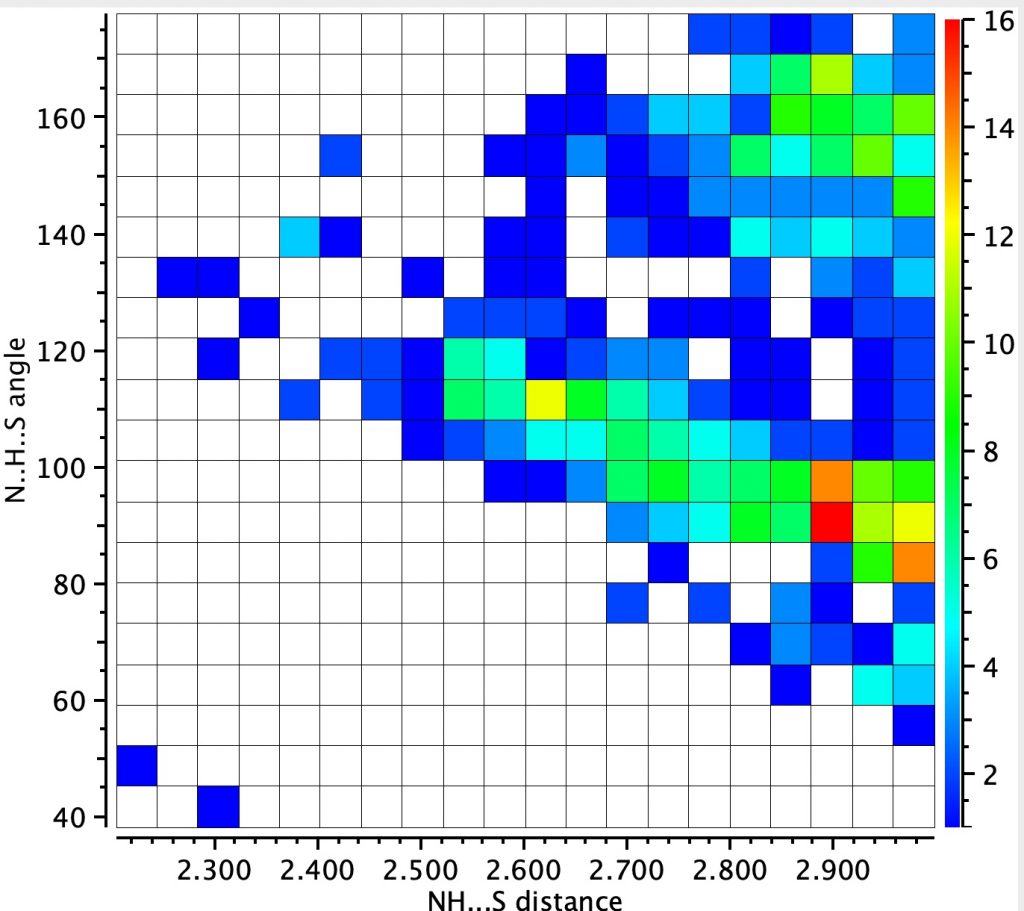
So we conclude there is ample evidence in small molecule crystal structures for the types of interaction mooted for Biotin with proteins.