The proposed identification of molecules with potential metal to carbon quadruple bonds, in which the metal exhibits trigonal bipyramidal coordination rather than the tetrahedral modes which have been proposed in the literature[1],[2],[3] leads on to asking whether simple trigonal coordination at the metal can also sustain this theme?
The rational for doing this is the observation for the trigonal bipyramidal molecules that repulsions between a non-bonding occupied d-orbital on the metal and one of the two putative metal to carbon σ-bonds resulted in the two electrons localising into a lone pair on carbon. By removing the electrons in this metal d-orbital or by increasing its size, the C σ-lone pair was encouraged to abandon some of its lone pair character and participate in a quadruple bond.
Another way of achieving this result is explored here, with the molecule shown above. Two of the trigonal carbon ligands are pinned back by the ring to reduce any potential repulsions. As before, this complex constitutes a filled 18-valence shell metal. The calculated orbitals (ωB97XD/Def2-SVPD, FAIR DOI: 10.14469/hpc/8206) are shown below.
| M=Co, r = 1.493Å. | |
|---|---|
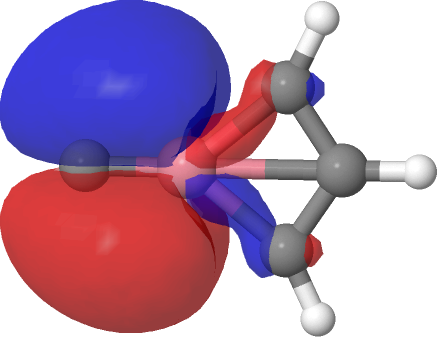
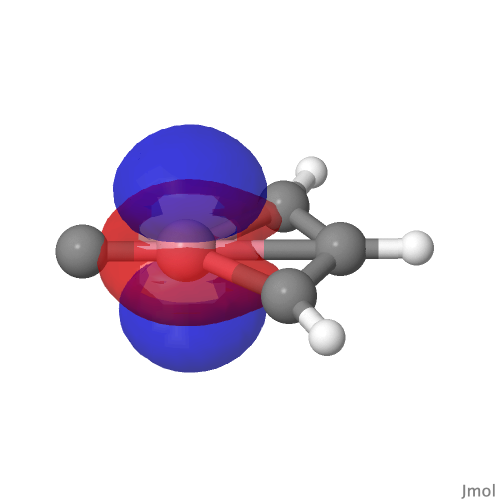
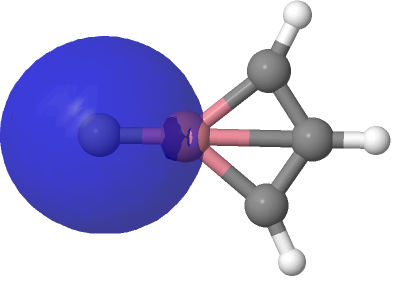
| NBO 26, π bond | NBO 24, non-bonding d-orbital |
 |
 |
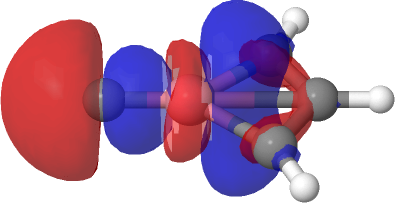
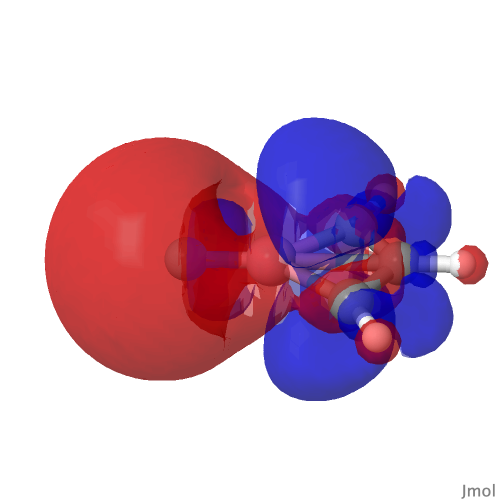
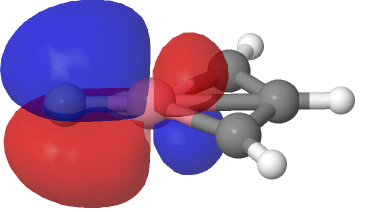
| NBO 23 σ bond, 0.02 au | NBO 23 σ bond, 0.01 au |
 |
 |
| NBO 22 π bond | NBO 14 σ bond |
 |
|
Despite the appearance of a bond between Co and C along the C⩸Co axis in the representations above (inserted off its own bat by the JSmol program), no such bond exists in the NBO list. No precedent for this kind of structure appears in the crystal structure database. As before, the NBO 23 σ bond at low isosurface thresholds expands to add a second layer along the Co⩸C axis, in which the nodal surface exists between the two layers rather than along the bond itself. It therefore constitutes a bonding orbital.
These quadruple bond motifs involving carbon are certainly starting to emerge in unexpected places and I do wonder how many more variations on this theme will be identified.
References
- A.J. Kalita, S.S. Rohman, C. Kashyap, S.S. Ullah, and A.K. Guha, "Transition metal carbon quadruple bond: viability through single electron transmutation", Physical Chemistry Chemical Physics, vol. 22, pp. 24178-24180, 2020. https://doi.org/10.1039/d0cp03436c
- A.J. Kalita, S.S. Rohman, C. Kashyap, S.S. Ullah, I. Baruah, L.J. Mazumder, P.P. Sahu, and A.K. Guha, "Is a transition metal–silicon quadruple bond viable?", Physical Chemistry Chemical Physics, vol. 23, pp. 9660-9662, 2021. https://doi.org/10.1039/d1cp00598g
- L.F. Cheung, T. Chen, G.S. Kocheril, W. Chen, J. Czekner, and L. Wang, "Observation of Four-Fold Boron–Metal Bonds in RhB(BO<sup>–</sup>) and RhB", The Journal of Physical Chemistry Letters, vol. 11, pp. 659-663, 2020. https://doi.org/10.1021/acs.jpclett.9b03484