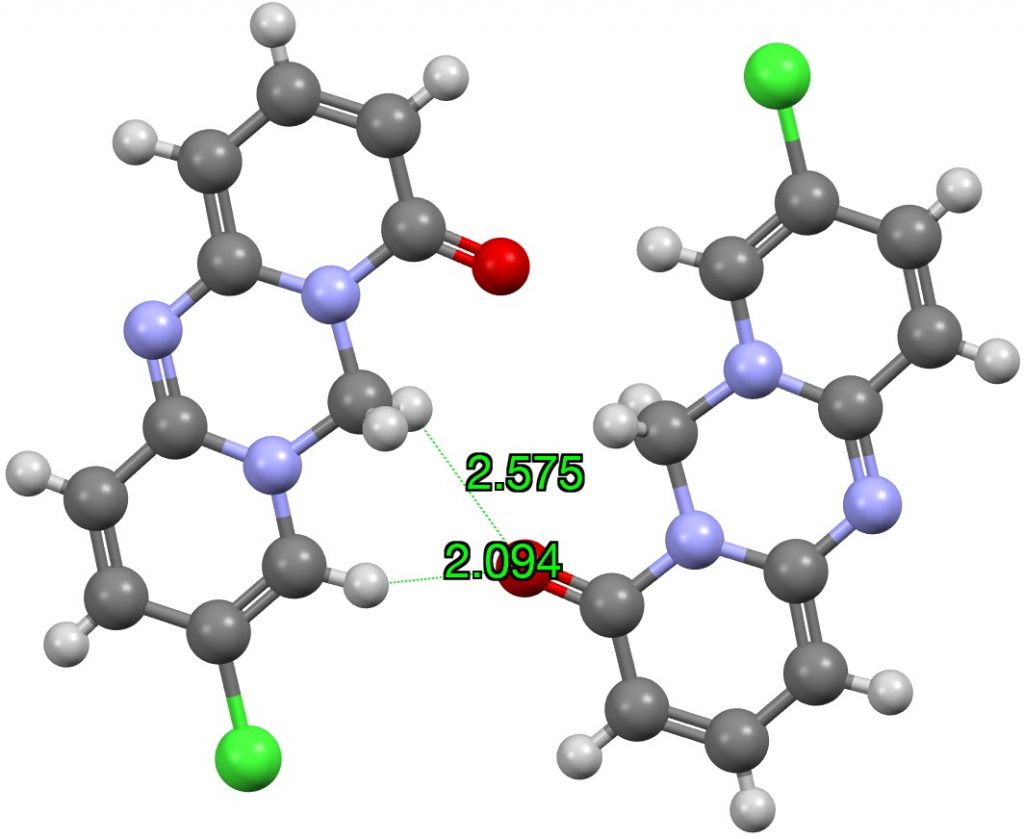
I have previously looked at the topic of hydrogen bonding interactions from the hydrogen of chloroform Here I generalize C-H…O interactions by conducting searches of the CSD (Cambridge structure database) as a function of the carbon hybridisation. I am going to jump straight to a specific molecule XEVJIR (DOI: 10.5517/cc5fgpq) identified from the searches appended to this post as interesting for further inspection.[1]
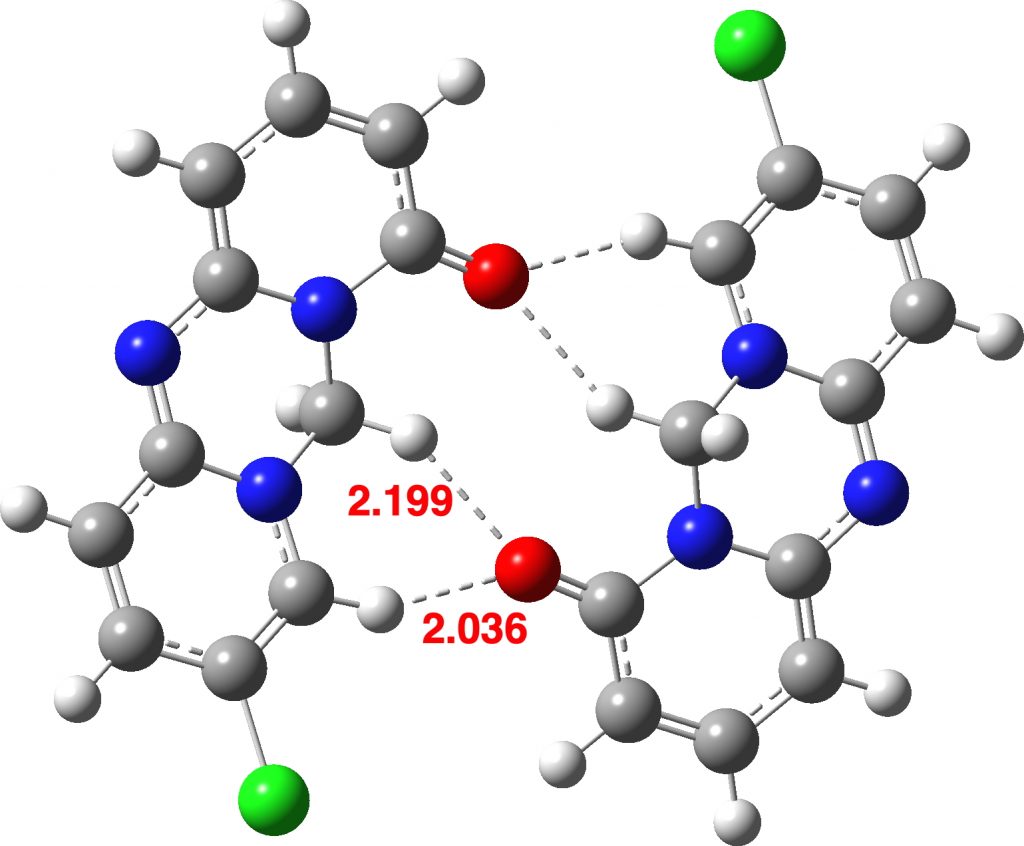
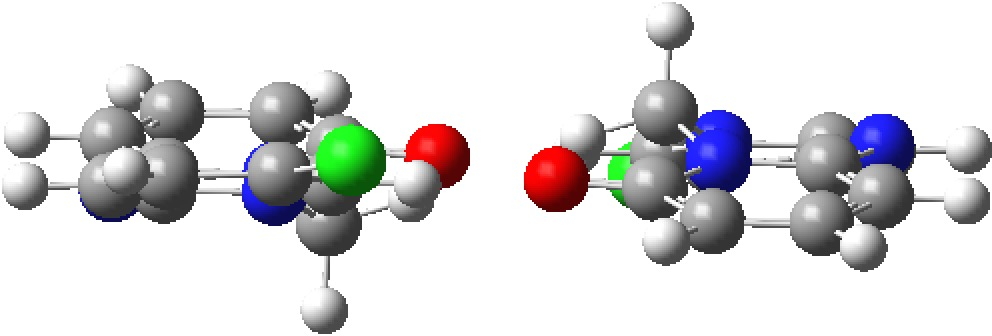
The distances from the carbonyl oxygen to CH groups of an adjacent intermolecular molecule are shown, revealing a bifurcated strong + weaker CH…O interaction. I would note that the CH…O distances are un-normalized, in the sense that a C-H distance obtained from X-ray diffraction data is normally about 0.1Å too short. A corrected value for the H…O distance is probably closer to 1.994Å. Next, a B3LYP+G3BJ/Def2-TZVPP calculation of just this dimeric interaction, which shows a somewhat different pattern, particularly from the carbonyl to the sp3-C-H (FAIR data DOI: 10.14469/hpc/5943) with one distance being shorter and one longer.
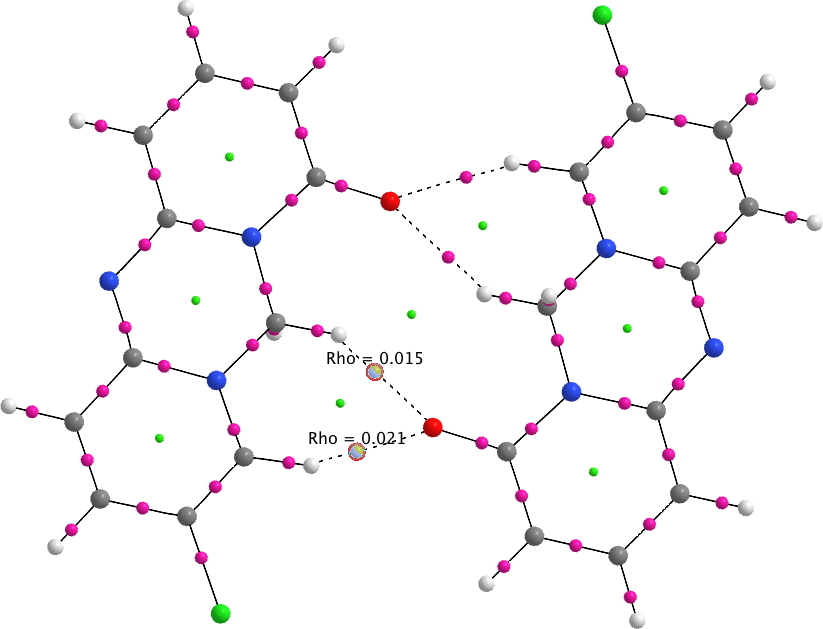
A QTAIM analysis reveals the electron density ρ(r) of 0.021au, a relatively high value indicating a relatively strong interaction.
Side-views reveals a possible reason for why the calculation does not match the crystal structure. In the crystal structure, the sp3-CH2 group adopts a different conformation from that computed for just two interacting molecules, since this shape allows more efficient stacking of layers and hence allowing stabilizing dispersion energy between the layers to overcome some loss of hydrogen bonding energies in the plane of the layer. If this packing constraint is removed in the pure dimer, one sp3-CH moves into the plane allowing a shorter interaction to the carbonyl oxygen and the other sp3-CH adopts a pure axial position, unconstrained by any packed layer above it.The absence of layered dispersion attractions is hence compensated by forming strong CH…O interactions.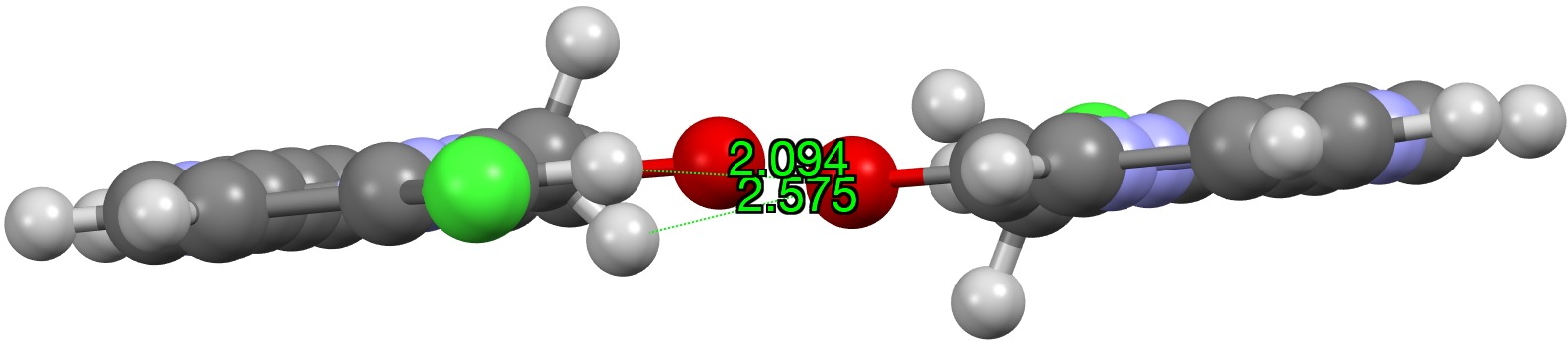
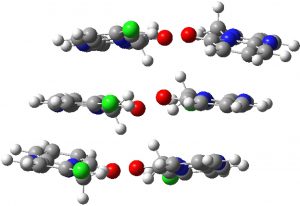
A calculation using six molecules arranged in three layers of two is an attempt to add back at least some of the layering dispersion terms (a full periodic boundary lattice calculation is the proper way of doing this calculation, but at the level chosen here would take far too much computer time!). The new CH…O distances are now 2.018 and 2.384Å (compared to 2.036 and 2.199Å for a model with just two molecules). Probably, more layers would be needed to replicate the crystal structure more accurately.
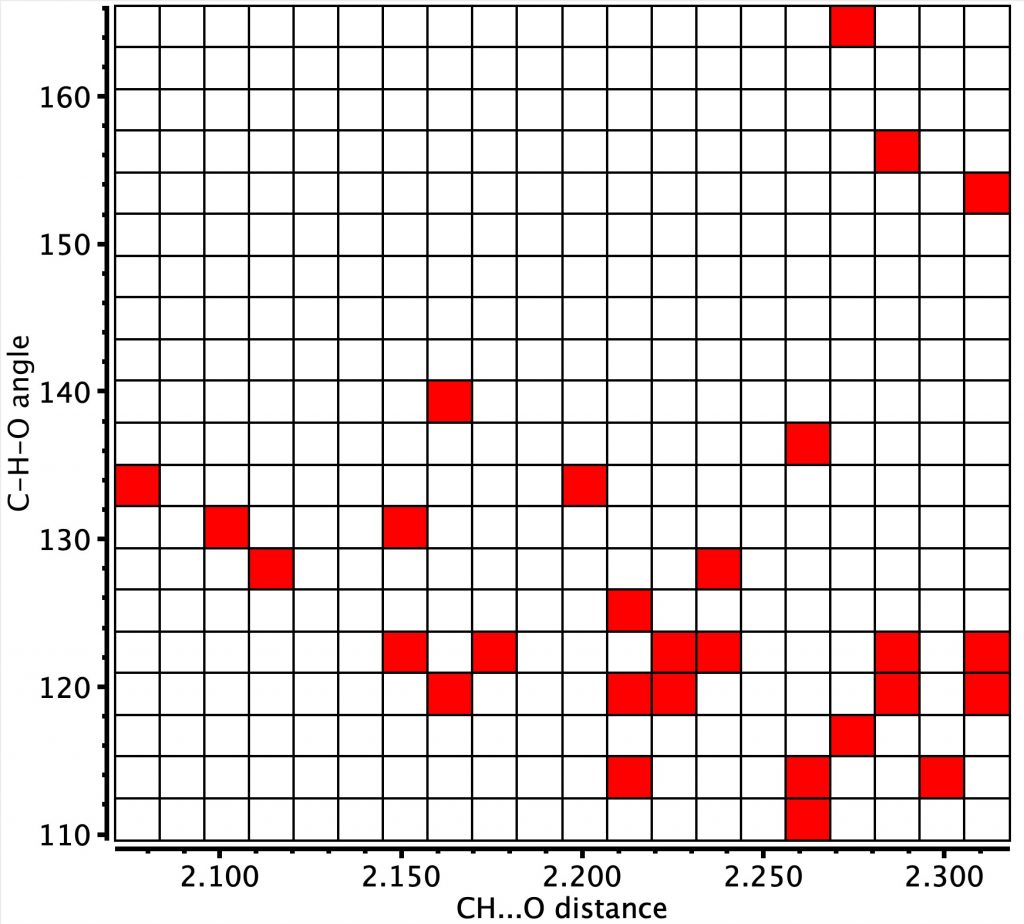
And now for the searches. The first is for sp-hybridised carbon, as an intermolecular interaction (R < 0.05, no errors, no disorder, T=<150K, H-position normalised for distances shorter than the sum of the vdW radii -0.4), for which a clear hot spot occurs at a H…O distance of ~2.1Å
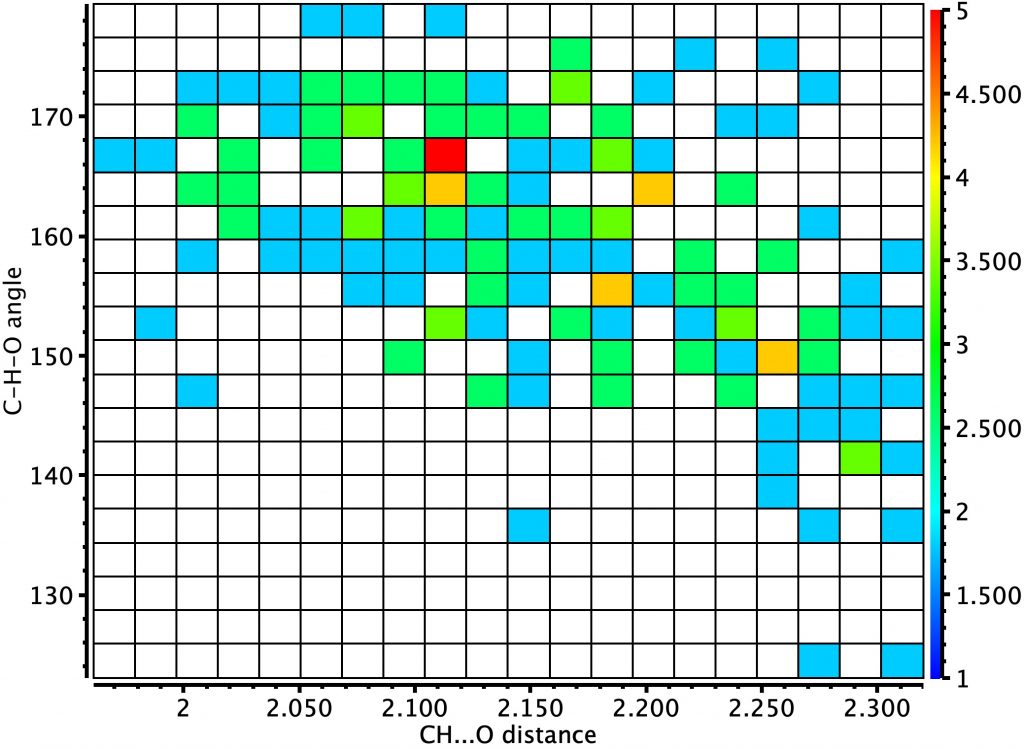
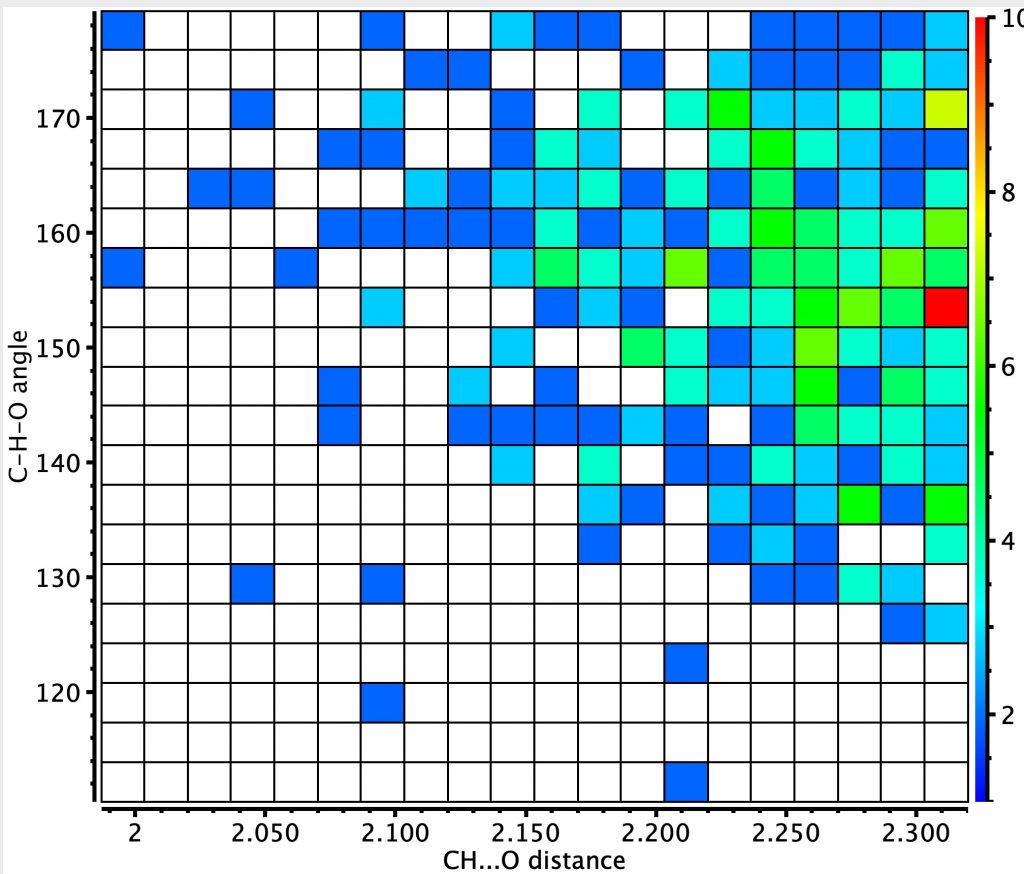
Next, sp2-C as an intermolecular interaction (T=<90K), where the hot spot is less distinct, being at the distance cut-off specified for the search. The shortest distance is ~2.0Å. I will return to this example shortly.
An intramolecular version of this search shows a clearer hotspot, again at ~2.15Å
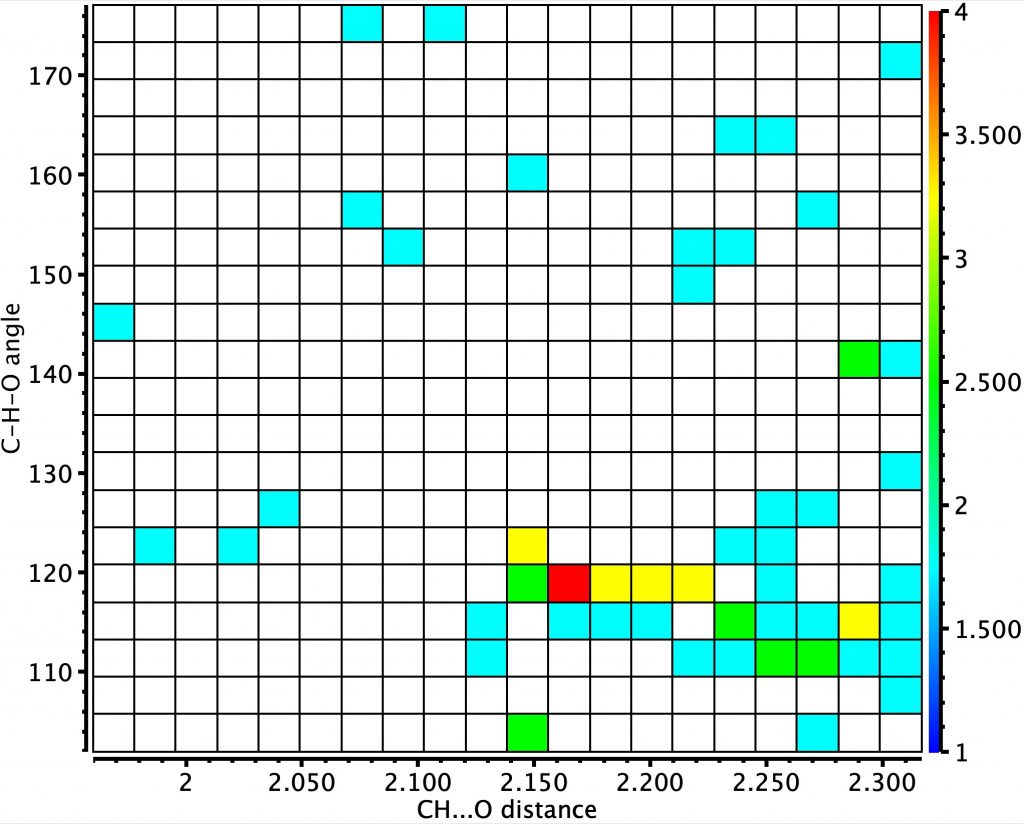
Next, intramolecular sp3 hybridisation, for which there few examples with no clear hotspot.
Finally, intermolecular sp3 hybridisation. The H…O distance hotspot is very slightly longer, as might be expected for a less acidic hydrogen. Nonetheless, the variation in the H…O distances with hybridisation is perhaps unexpectedly small.
To summarise, by performing a general search of the crystal structure database, one can identify general trends and then go to inspect outliers. In this case, this brought the focus onto an (dare I say otherwise umremarkable) molecule in which layers of aromatic molecules set up a competition between intra-layer CH…O hydrogen bonding and inter-layer dispersion stabilizations. I suspect this competition between these two type of weak interactions is far more common than is generally recognised.
References
- K.S. Huang, M.J. Haddadin, M.M. Olmstead, and M.J. Kurth, "Synthesis and Reactions of Some Heterocyclic Azacyanines<sup>1</sup>", The Journal of Organic Chemistry, vol. 66, pp. 1310-1315, 2001. https://doi.org/10.1021/jo001484k