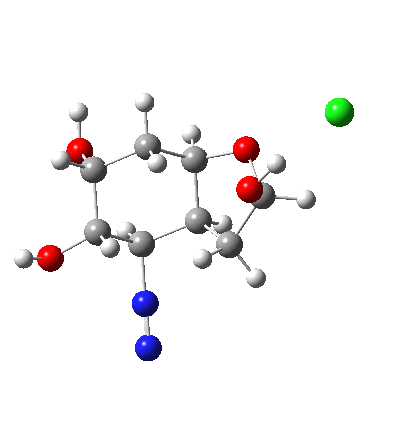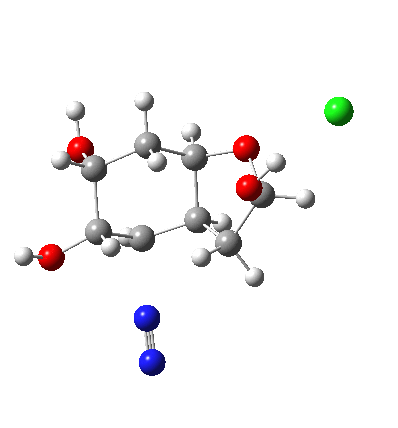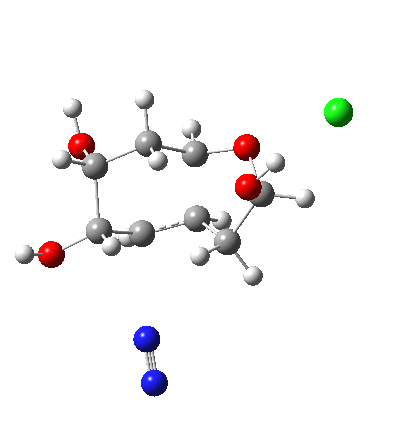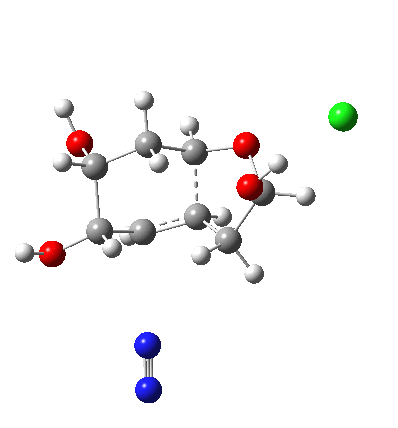
In answering tutorial problems, students often need skills in deciding how much time to spend on explaining what does not happen, as well as what does. Here I explore alternatives to the mechanism outlined in the previous post to see what computation has to say about what does (or might) not happen.
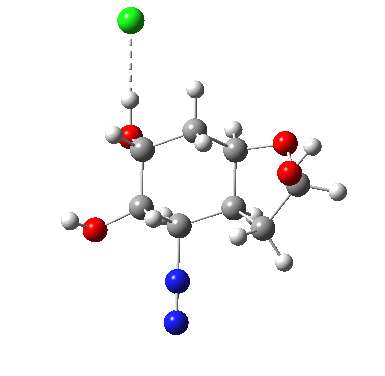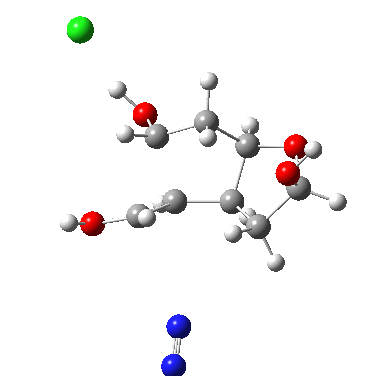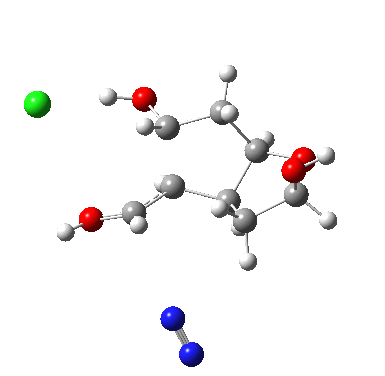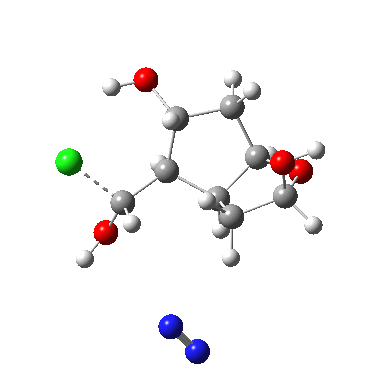
I start with posing the question what does the chloride counter-ion do? If you are aware of the literature on computational reaction mechanisms, you may note that where ionic species are involved, one of the ions is often excluded from the calculations. Here for example, the pertinent reacting species is a diazonium cation, but the anion would likely not be mentioned, and the calculation would be performed as a charged cation (the physically unrealistic charge=1 in the input file!). This is because of an awkward difficulty with ion-pairs. There is no formal bond between the two charged fragments (unless a zwitterion) and so it is not entirely obvious quite where to place the counter-ion. In the diagram above, position 1 is where it was in my first exploration, but with knowledge that it might form a hydrogen bond to an acidic hydrogen, one could also perhaps place it into positions 2 or 3. In 2, as shown by the blue arrows and product above, an entirely different reaction occurs known as the Grob fragmentation.[1] In fact as a di-carbonyl compound, it can then participate in an acid-catalysed aldol condensation and this can lead to the same product as the original Tiffeneau-Demjanov rearrangement, albeit with loss of stereochemical integrity. So it might be worth effort in explaining whether this alternative is likely (in other words how robust the likely stereochemical integrity of the product is).
| System | Relative TS free energy | TS Dipole moment | DataDOI |
|---|---|---|---|
| 1 | 0.0 | 17.7 | [2] |
| 2 | 1.4 | 24.2 | [3] |
| 3 | 3.7 | 29.3 | [4] |
The energies of the three located transition states increase with the dipole moment; as the counter-ion moves further from the positive charge, its position becomes less stable. Still, route 2 is not that much higher in energy. Time for an IRC (intrinsic reaction coordinate) to explore what actually does happen during route 2, the possible Grob rearrangement.
The reaction animation above shows the required Grob characteristic, the green bond breaking. But instead of the OH then de-protonating, the hydrogen stays in place and instead the Tiffeneau-Demjanov migration takes place. This will require removal of a different proton and indeed in the latter stages, the chloride anion starts off in its determined journey to do so.
The variation in dipole moment as the reaction proceeds is fascinating. At IRC -6, it reaches a minimum, but then reverses itself in hunt of a better way of reducing the dipole moment.
What about 3? This is slightly artificial, since the real system has a methoxy group here, which would inhibit this route. One can still learn chemistry though. The hydrogen bond formed from chloride to the OH encourages the anomeric effect to form a partial oxy-anion, which in turn encourages the red bond to break rather than the green one. But in fact no complete proton transfer happens, and the reaction reaches a non-productive cul-de-sac.
So, to conclude, there is no Grob fragmentation! Instead, a slightly confused Tiffeneau-Demjanov migration occurs in a rather more roundabout manner than previously. We have explored here just TWO reaction trajectories. A more statistical exploration of the trajectory landscape will give us a more complete picture, but I rather fancy that would be very well above the call of duty required to answer a stereochemical problem!
References
- C.A. Grob, and W. Baumann, "Die 1,4‐Eliminierung unter Fragmentierung", Helvetica Chimica Acta, vol. 38, pp. 594-610, 1955. https://doi.org/10.1002/hlca.19550380306
- H.S. Rzepa, "C 8 H 13 Cl 1 N 2 O 4", 2015. https://doi.org/10.14469/ch/191653
- H.S. Rzepa, "C 8 H 13 Cl 1 N 2 O 4", 2015. https://doi.org/10.14469/ch/191654
- H.S. Rzepa, "C 8 H 13 Cl 1 N 2 O 4", 2015. https://doi.org/10.14469/ch/191655
Tags: animation, Chemical bond, condensation, Demjanov rearrangement, energy, Rearrangement reactions, Tiffeneau–Demjanov rearrangement