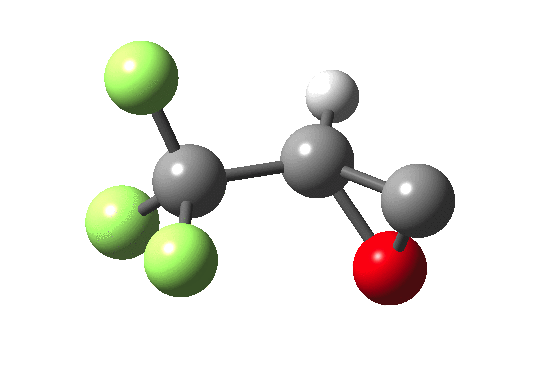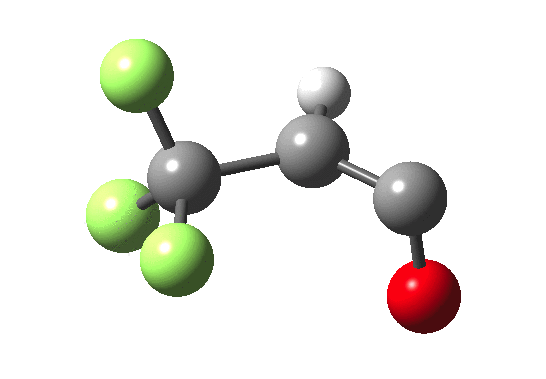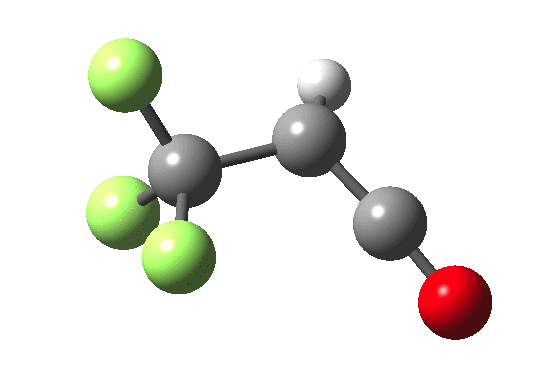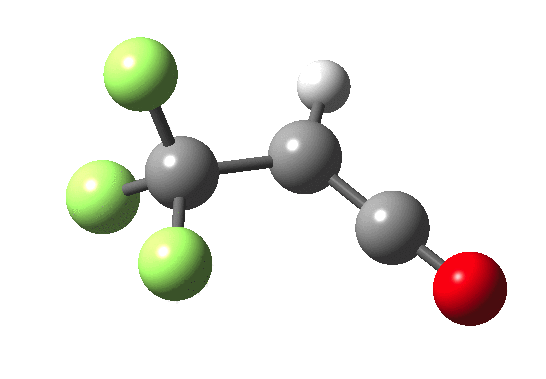
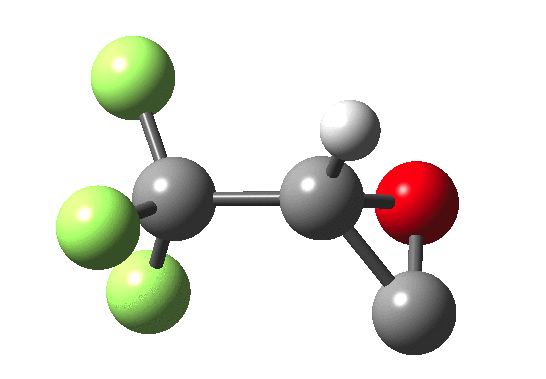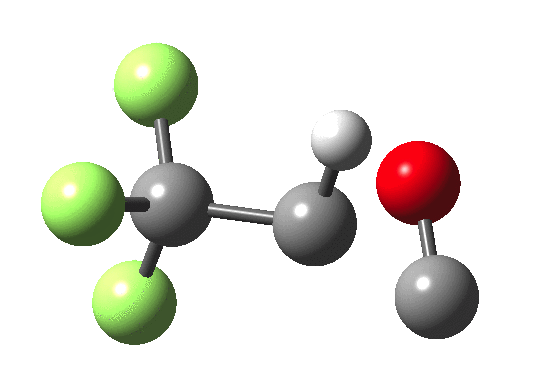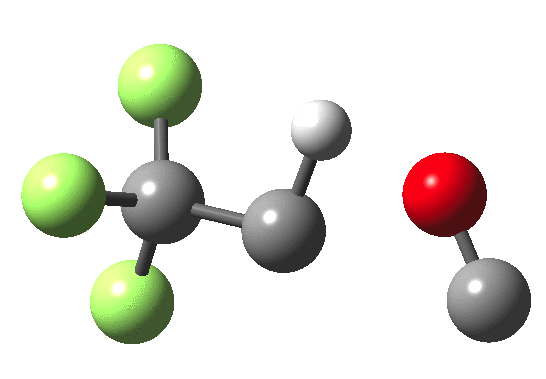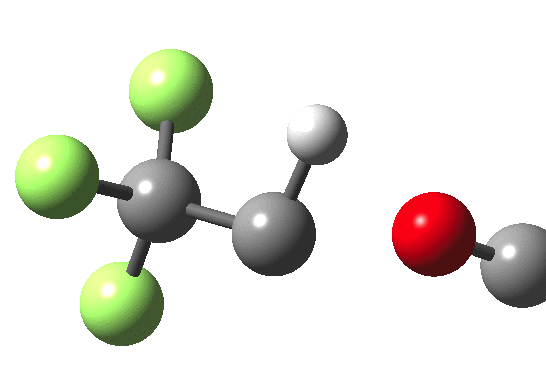
How does an anaesthetic work? Surprisingly, it is only recently[1] that the possible binding sites of the anaesthetic propofol (2,6-di-isopropylphenol) have been identified using a technique known as photoaffinity labelling.[2] A propofol analogue was constructed[1] by replacing one of the isopropyl groups with a trifluoromethyl diazirine group (R=CF3, X=Y=N below). Upon photolysis, this species looses nitrogen and forms a carbene as a reactive species, which with further chemistry binds covalently[2] to adjacent amino acids in the binding pocket.These modified segments could then be analysed by mass spectrometry.[1] An isomer of diazirine is diazomethane, which is some 11 kcal/mol lower in free energy, but fortunately the diazirene is preventing from thermally isomerising to this species by a large kinetic barrier. That was the intro; now for a connection.‡ I recently attended a presentation on another medical topic, the therapeutic uses of carbon monoxide.[3] In higher concentrations it is notoriously lethal, but with appropriate delivery it can be therapeutic. So, intertwingling, I asked myself what the properties of the carbon monoxide isoelectronic analogue of a diazirine might be (X=C, Y=O below).
Firstly, a search using Scifinder. More than 102 million molecules and substances are reported there. Of course, that does not mean the molecule actually exists, since many structures are only known by calculation! But I could not find the CO analogue using a sub-structure search. So this simple isoelectronic analogue of a very well-known molecule type is not apparently well-known then (although I feel sure that a theoretical chemist must have calculated it at some stage,† even if the results were not reported and abstracted by CAS). Here is a ωB97XD/6-311G(d,p)/SCRF=nitromethane set of calculations (kcal/mol).
| Species | ΔΔG298 ring | ΔΔG298 linear | ΔG† XY extrusion | ΔG† ring opening |
|---|---|---|---|---|
| X=Y=N | 0.0[4] | -11.0[5] | 36.1[6] | — |
| X=C, Y=O | 0.0[7] | -63.3[8] | 27.3[9] | 46.8[10] |
We learn the following:
- The CO analogue is far less stable as a ring than the NN equivalent, but it is a clear-cut minimum in the energy surface.
- In the absence of any other conditions (for example protons), the free energy barrier for extruding CO is actually quite high, being only a little lower than the diazirine itself. The latter is thermally reasonably stable and not a fragile molecule at all. At face value therefore if made, such a cyclo-oxenium ylid might have a long enough “shelf life” to be detectable or trappable, certainly at lower temperatures.
- It shows an even higher barrier to ring opening to form the far more stable ketene. This barrier is high enough that this reaction probably involves e.g. biradicals or other pathways.
The question arises how such a CO analogue of diazirines might be made. The very high relative energy does tend to suggest it might have to be a photochemical approach. This would be a challenge, given that photochemistry is also likely to promote CO extrusion. Most likely this species is destined to join the (increasing) collection of molecules known to science only by their computed properties!
‡Such connectedness in human knowledge has been termed intertwingularity by Ted Nelson.
†Scifinder by and large only abstracts articles published in journals, along with some of the data found there. Most calculations never make it to such articles. The newly topical area of Research Data Management strives to put in place an infrastructure that addresses such issues.[11]
References
- G.M.S. Yip, Z. Chen, C.J. Edge, E.H. Smith, R. Dickinson, E. Hohenester, R.R. Townsend, K. Fuchs, W. Sieghart, A.S. Evers, and N.P. Franks, "A propofol binding site on mammalian GABAA receptors identified by photolabeling", Nature Chemical Biology, vol. 9, pp. 715-720, 2013. https://doi.org/10.1038/nchembio.1340
- L. Dubinsky, B.P. Krom, and M.M. Meijler, "Diazirine based photoaffinity labeling", Bioorganic & Medicinal Chemistry, vol. 20, pp. 554-570, 2012. https://doi.org/10.1016/j.bmc.2011.06.066
- R. Motterlini, and L.E. Otterbein, "The therapeutic potential of carbon monoxide", Nature Reviews Drug Discovery, vol. 9, pp. 728-743, 2010. https://doi.org/10.1038/nrd3228
- H.S. Rzepa, "C 2 H 1 F 3 N 2", 2015. https://doi.org/10.14469/ch/191529
- H.S. Rzepa, "C 2 H 1 F 3 N 2", 2015. https://doi.org/10.14469/ch/191530
- H.S. Rzepa, "C2HF3N2", 2015. https://doi.org/10.14469/ch/191534
- H.S. Rzepa, "C 3 H 1 F 3 O 1", 2015. https://doi.org/10.14469/ch/191531
- H.S. Rzepa, "C 3 H 1 F 3 O 1", 2015. https://doi.org/10.14469/ch/191535
- H.S. Rzepa, "C3HF3O", 2015. https://doi.org/10.14469/ch/191532
- H.S. Rzepa, "C3HF3O", 2015. https://doi.org/10.14469/ch/191533
- H. Rzepa, "Workshop presentation on Research Data Management at Imperial College London, 29th September, 2015.", 2015. https://doi.org/10.14469/hpc/143
Tags: anaesthetic, Diazirine, GABAA receptors, photo affinity labelling, propofol