An article entitled "Four Decades of the Chemistry of Planar Hypercoordinate Compounds"[1] was recently reviewed by Steve Bacharach on his blog, where you can also see comments. Given the recent crystallographic themes here, I thought I might try a search of the CSD (Cambridge structure database) to see whether anything interesting might emerge for tetracoordinate carbon.
The search definition is shown below using a simple carbon with four ligands,‡ the ligands themselves also being tetracoordinate carbon. The search is restricted to data collected below temperatures of 140K, as well as R-factor <5%, no errors and no disorder. Cyclic species are allowed and a statistically reasonable 2773 hits emerged from the search.
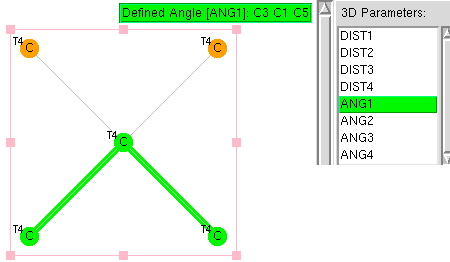
Recollect that the idealised angle subtended at the centre is 109.47°. I show below three separate heat plots of the search results. Why three? The way the search software (Conquest) works is that one could define four C-C distances and six angles, and then plot any combination of one distance and one angle. I show just three combinations here, but could have included many more.
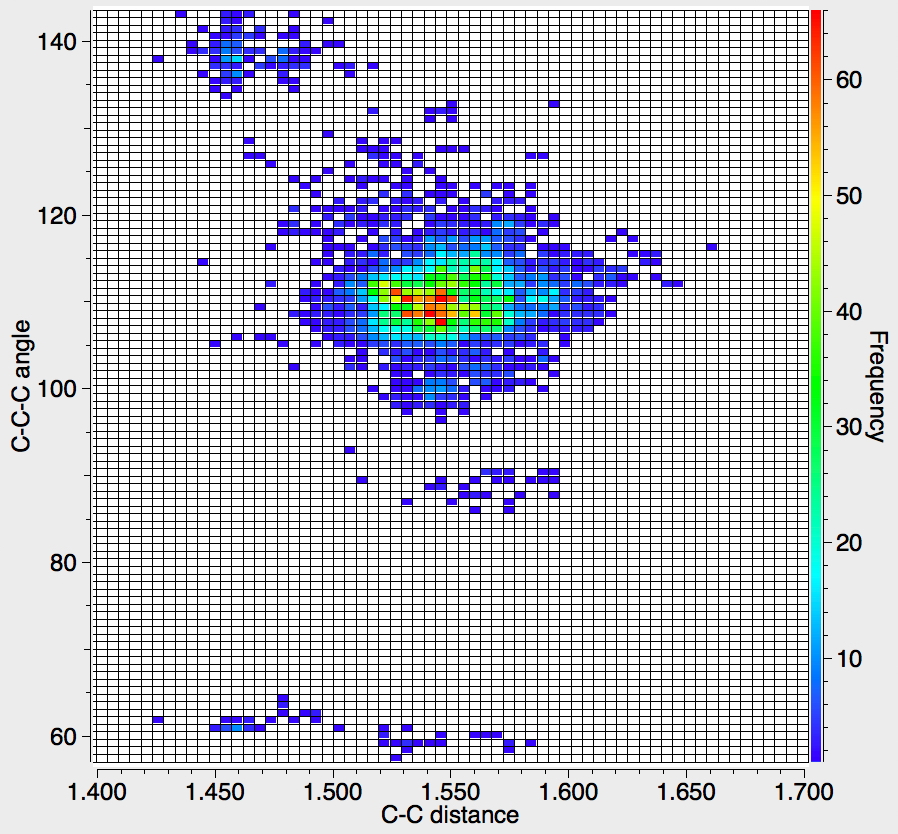
There appear to be four distinct clusters of values for this angle that emerge from the three plots shown below (the "bin size" is 100, and the frequency colour code indicates how many hits there are in each bin).
- The hotspot is unsurprisingly ~109° with a corresponding C-C distance of ~1.54Å.
- There may be two clusters at angles of ~60° (cyclopropane), with C-C values ranging from ~1.47 to ~1.55Å.
- A collection at ~90° (mostly cyclobutane?), with C-C values up to 1.6Å.
- A collection at ~140° (again small rings), now with much shorter C-C values of ~1.46Å. This reminds of the approximation that the hybridisation in e.g. cyclopropane is a combination of sp5 and sp3.
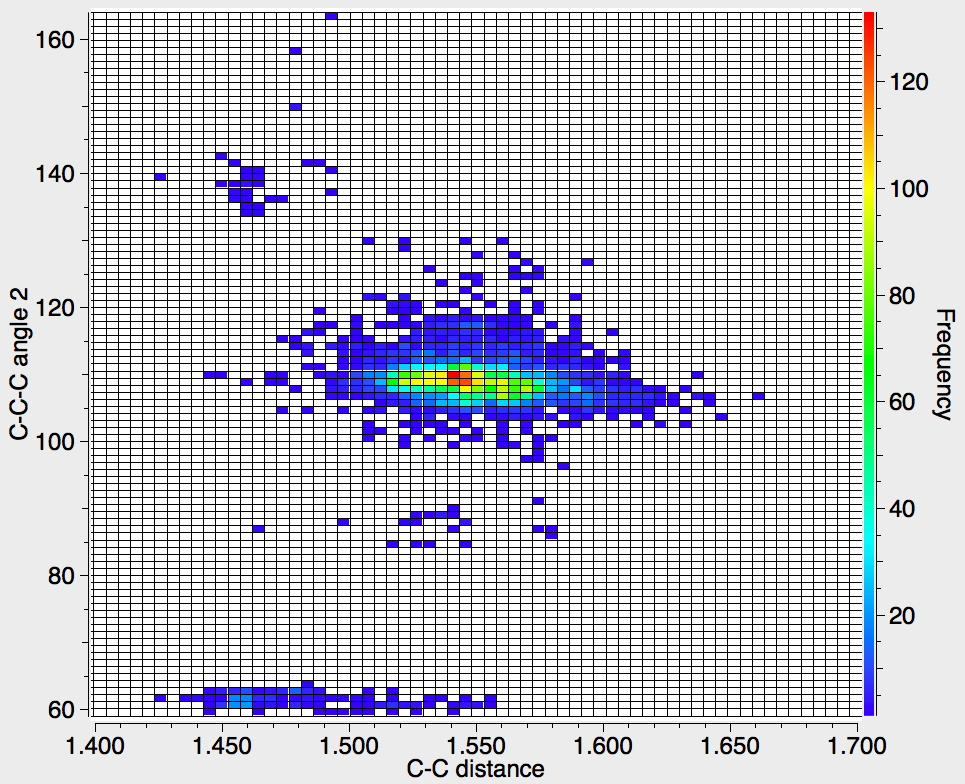
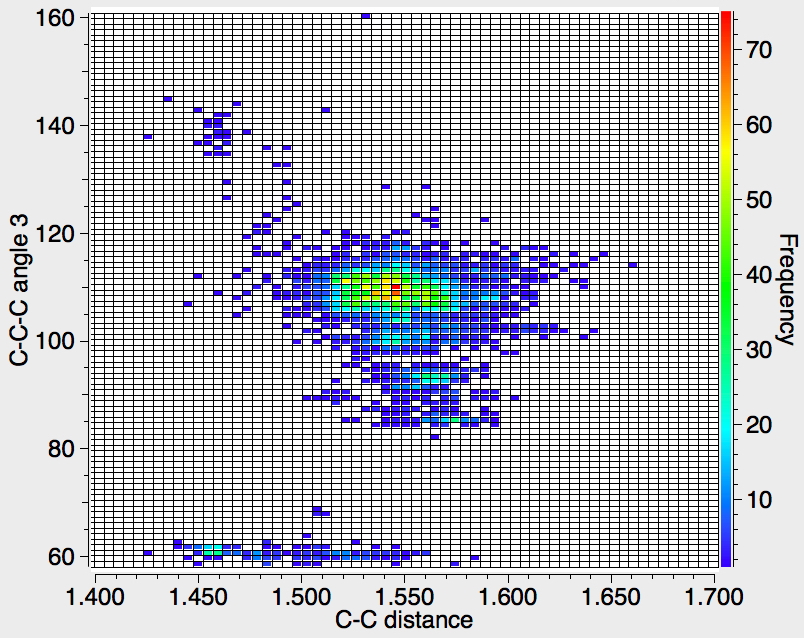
Ideally, what one might want to plot would be sums of four angles; for a pure tetrahedral carbon the sum would always be 438° (4*109.47°) but for a pure planar carbon it could be as low as 360° (4*90°). One could then see how closely the distribution approaches to the latter and hence reveal whether there are any true planar tetracoordinate carbon species known. Although the Conquest software cannot analyse in such terms, a Python-based API has recently been released that should allow this to be done, although I should state that this requires a commercial license and it is not open access code. If we manage to get it working, I will report!
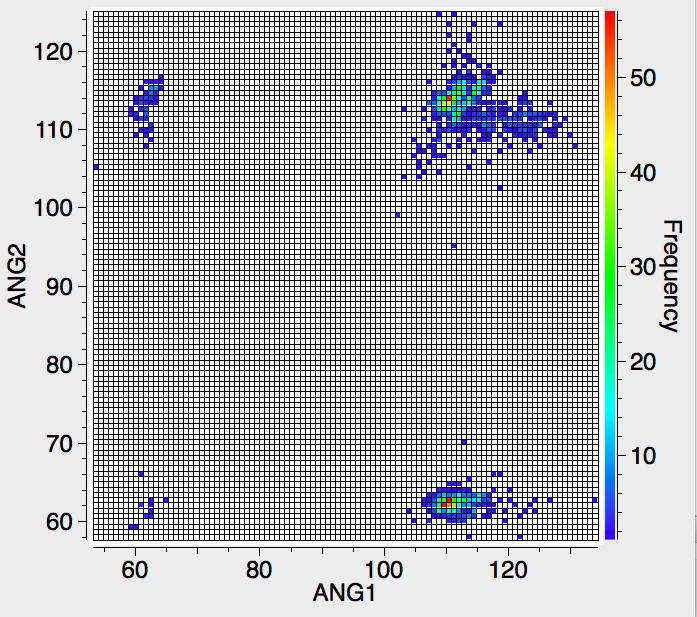
‡As a teaser I also include a plot of six-coordinate carbon, in which the ligands can be any non-metal. Note the clusters at angles of 60, ~112 and ~120-130°. It is worth pointing out that the definition of the connection between a carbon and a ligand as a "bond" becomes increasingly arbitrary as the coordination becomes "hyper". Because crystallography does not measure electron densities in "bonds", we know nothing of its topology in this region. It is therefore quite possible that the appearance of the heat plot below might be related just as much to whatever convention is being used in creating the entry in the CSD as it would be to a quantum analysis of the bonding.
Acknowledgments
This post has been cross-posted in PDF format at Authorea.
References
- L. Yang, E. Ganz, Z. Chen, Z. Wang, and P.V.R. Schleyer, "Four Decades of the Chemistry of Planar Hypercoordinate Compounds", Angewandte Chemie International Edition, vol. 54, pp. 9468-9501, 2015. https://doi.org/10.1002/anie.201410407
Tags: Angle, Cambridge, chemical bonding, Cycloalkane, Cyclopropane, HTML, HTML element, Ligand, metal, search definition, search results, search software, Steve Bacharach