I have several times used arrow pushing on these blogs. But since the rules for this convention appear to be largely informal, and there appears to be no definitive statement of them, I thought I would try to produce this for our students. This effort is here shared on my blog. It is what I refer to as the standard version; an advanced version is in preparation. Such formality might come as a surprise to some; arrow-pushing is often regarded as far too approximate to succumb to any definition, although it is of course often examined.
- How the conventions arose
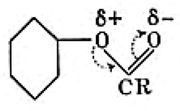
- These were established largely by textbook authors. The first with a noticeably modern look was Hunter (1934). Here he is explaining using his notation why an ester group is meta-directing towards aromatic electrophilic substitution. The convention of the time was to represent benzene as a simple hexagon, without the additional bonds
-
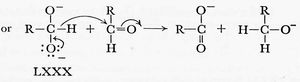
Gould in 1959 (“Mechanism and structure in organic chemistry”, reviewed[1] adopted a clearly modern form. In this example, we have a curly arrow starting at the mid-point of a C-H (hydride) bond, and ending at the nucleus of (an electrophilic) carbon atom. His arrows also start at lone pairs rather than negative charges, but at some stage the convention has evolved to dispense with the : indicating a lone pair, and to start the arrow at the charge instead.
-
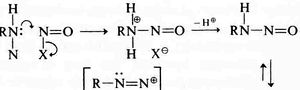
Sykes (“A guidebook to mechanism in organic chemistry” in 1961, reviewed [2] is very similar to Gould, but in his example he shows a nitrogen lone pair heading towards the mid-point of a N-N forming bond, rather than ending at the nucleus of the (electrophilic) nitrogen atom as Gould would have done. Nowadays, we clarify Sykes convention a bit further by adding a dotted line to the forming bond so that the arrow can both start and end on either a lone pair or a line. This dotted line is distinct from dotted or dashed lines used to represent resonance.
- These were established largely by textbook authors. The first with a noticeably modern look was Hunter (1934). Here he is explaining using his notation why an ester group is meta-directing towards aromatic electrophilic substitution. The convention of the time was to represent benzene as a simple hexagon, without the additional bonds
- The rules :This set can be referred to as following the Sykes convention, and its main points are summarised here:
- There are two main types of mechanistic arrows, linear and cyclic (there is a very rare third type[3]. The former have a one clear start and end, the latter can circulate in two directions (clockwise or anticlockwise).
- Some reactions may involve using a combination of linear and cyclic arrows (for example the bromination of an alkene or alkene epoxidation by peracid).
- The most common mechanism (non-radical) involves just a single arrow either originating or ending at a bond/atom. Normally, no pair of atoms undergo a bond order change between them of more than one.
- There are rare exceptions involving two, or even three arrows starting or ending at a bond). The bond order for these can involve changes of 2 or even 3.
- Arrows will start at a centre with readily released electrons (nucleophilic for linear reactions). Types of readily released (nucleophilic or nucleus seeking) electron pairs are:
- Lone pairs ( : ) associated with an atom. Here, the order of nucleophilicity is C > N > O > F for the first row. The arrow by convention starts at the :.
- Bonds. We have to take into account the type of bond.
- σ-bonds. Such electron pairs are relatively non-nucleophilic (the s-character of the bond orbital is high) and so only bonds to less electronegative elements can release electrons. Thus a B-H bond can release an electron pair more readily than a C-H bond (in both cases this is called a hydride transfer). Another type of σ-bond which can more easily release electrons is that of cyclopropane (largely because the degree of s-character is lower than a normal σ-bond).
- π-bonds. Because these involve only p-AOs (no s-character) they can release electrons relatively easily. Again, this release is easier with less electronegative elements; (B=B) > C=C > C=N > C=O.
- δ-bonds, as found in high-bond order metal-metal bonds. Very rarely used in arrow pushing.
- Arrows will end at electron accepting sites (electrophiles), to either form a lone pair or a new bond.
- The arrow can end at an : associated with an atom. The order of electrophilicity is Halogen > O > N > C > B.
- The arrow can end at a bond. Again, a new σ-bond (with high s-character) is a better acceptor of electrons than a π-bond (no s-character), and new bonds associated with more electronegative atoms are the better acceptors. A (formal) positive charge on an atom helps make it a good acceptor (such as a carbocation).
- There are four potential combinations of the above rules:
- Bond → bond
- Bond → lone pair
- Lone pair → bond
- Lone pair → lone pair. This latter is very rare.
- The symmetry of the electrons involved must conform to group theory/symmetry. For example, if the reactant and product of a reaction maintain a plane of symmetry which allows one to distinguish between π- and σ-electrons, one cannot convert a π-pair into a σ-pair during the reaction (or vice versa) if its group-theoretical symmetry has to change. An example of falling foul of this rule is in fact the very first arrows ever pushed in the literature! An elaboration of this rule is used to define whether any particular pericyclic reaction (a reaction with cyclic arrows) is allowed or forbidden.
- The convention above makes no attempt to imply symmetry, and as such therefore can result in incorrect mechanisms, as noted above. There are no plans at the moment to add symmetry notation to arrow pushing.
- The coordinates of the arrows. This has in the past been very imprecisely defined, but having a precisely defined start and end for each (double-headed, electron pair) arrow could be regarded as being helpful. It is also ascertainable:
- Arrows starting or ending at bonds. These coordinates can be computed from the topology of the electron density of either the reactant (the starting point) or the product (the arrow endpoint). Electron density is an experimental observable (using e.g. crystallography) as well as a computable property using quantum mechanics. Its topology (curvatures if you like) can be obtained by appropriate analysis. The key topological property is the bond-critical-point or BCP, which generally can be located at approximately the mid-point of the line connecting the two nuclei (its precise position depends on the relative electronegativities).
- Arrows starting or ending at lone pairs (:). Here too topological analysis of the electron density can result in defining the centroid of a lone pair, with again precise coordinates.
-
Practically, no-one is ever going to perform topological analysis of the electron density in order to push arrows! So a good approximation is to assume that a BCP is located at the mid-point of a bond and a lone pair is located at an atom (mindful this is NOT coincident with the nucleus, since we know a lone pair has p-character). This approximation leads directly to the Sykes convention.
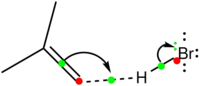
- These points can be summarised in the diagram above, involving reaction between butene (as the electron releasing molecule) and HBr (as the electron accepting molecule).
- The green dots represent mid-points of bonds (either breaking or making), and more formally correspond to the BCPs described above.
- The green : represents a lone pair being formed, more formally corresponding to the lone pair centroid.
- A dotted line is drawn to the forming bond. This is not strictly part of the Sykes convention; it can be optionally omitted and left as implied (in much the same way that most hydrogen atoms in molecules are implied).
- There are two (optional) red dots also shown. These are another convention which here is explicit, but is often left implicit. One can regard the red dots as the location of hinges, and regard the arrows as rotating about these hinges. A metaphor might be a hinged door, which is opening (bond breaking) by rotating around one hinge, and closing (bond or lone pair forming) by rotating about the next hinge. In this metaphor a covalent bond is a closed-door and a lone pair is an open door. Adding these hinges allows one to define a simple checking-rule.
- For reactions where no atom undergoes a valency change, the hinges MUST be located on alternating atoms. No two adjacent atoms can have hinges.
- An exception might be where linear and cyclic arrows are mixed.
- For reactions where one atom undergoes a valency change (the most common examples are 4-valent carbon changing to 2-valent carbon, ie a carbene, or 3-valent nitrogen forming 1-valent nitrene, but it also includes changes in oxidation states of transition metals etc), there must be one occurrence of adjacent atoms (ie bonded atoms) each having a hinge.
- For reactions where no atom undergoes a valency change, the hinges MUST be located on alternating atoms. No two adjacent atoms can have hinges.
- Most reactions involve more than one arrow (electron pair). The question can then arise as to the relative timing of the various arrows.
- If no explicit intermediate is involved, the arrows are said to be concerted, they all operate at the same time.
- Any concerted reaction however need not be synchronous, ie the arrows need not all occur at exactly the same time. Sometimes, the arrows can occur in phases.
- To determine either the concertedness or synchronicity of any arrow pushing mechanism is however way beyond our current ability to measure (although there are prospects of doing so). Such properties can be computed, but again doing so requires a very sophisticated calculation. Even if these properties can be ascertained, representing them in the convention shown above is also going to be a challenge. So these attributes are currently not attempted using the conventions above.
- Radical reactions. These differ from the electron pair reactions since one arrow is assigned to each electron.
- Normally, all the arrows used are single-electron fish-books, but there are some rare cases where both fish-book and normal arrows can be combined (the Birch reduction for example).
- In general two fish-hook arrows from different sources will both head off to a bond-mid-point (the BCP of the forming bond).
- Although the fish-hook implies an electron spin, there is no convention to ensure that the spin-pairing in any formed new bond is correct (strictly, two fish-hooks of opposite spin should combine).
- Because two fish-hook arrows derive from an electron pair, there is no sense of direction (the two arrows head off in opposite directions). Radical arrows tend not to be nucleophilic/electrophilic.
- Arrows for reactions involving excited states (photochemistry). These are by and large regarded as beyond the scope of arrow pushing, although one could regard them as triplet state reactions involving fish-hook arrows.
- There are two main types of mechanistic arrows, linear and cyclic (there is a very rare third type[3]. The former have a one clear start and end, the latter can circulate in two directions (clockwise or anticlockwise).
The rules above are terse, and in particular I have not tried to add more than one example, although quite a number are sprinkled throughout this blog.
References
- W.M. Schubert, "Mechanism and Structure in Organic Chemistry (Gould, Edwin S.)", Journal of Chemical Education, vol. 37, pp. 379, 1960. https://doi.org/10.1021/ed037p379.2
- D.F. Detar, "A guidebook to mechanism in organic chemistry (Sykes, Peter)", Journal of Chemical Education, vol. 40, pp. A224, 1963. https://doi.org/10.1021/ed040pa224.1
- B.S. Young, R. Herges, and M.M. Haley, "Coarctate cyclization reactions: a primer", Chemical Communications, vol. 48, pp. 9441, 2012. https://doi.org/10.1039/c2cc34026g
Thanks for the analysis, Henry. I discovered how intricate the rules of electron-flow arrows are when I wrote an algorithm for calculating the products of e-flow arrows acting on one or more compounds. The algorithm turned out to be remarkably complex, especially when it had to handle errors that students made in their arrow-drawing, such as an arrow starting at H(+). Here is an alternative analysis from yours.
1. An electron-flow arrow can represent the movement of one or two electrons.
2. An electron-flow arrow can begin at either an atom or a bond, called the electron source. (In the first case, some chemists prefer to show the arrow beginning at an unshared electron or lone pair of the source atom, rather than at the atomic symbol itself, but the two representations have the same meaning.)
3. An electron-flow arrow can end at an atom, an existing bond, or an incipient bond between two atoms, called the electron sink.
4. An electron-flow arrow originating at an atom A can point to another atom B if and only if the two atoms do not already share a bond.
4a. If the electron-flow arrow is a two-electron arrow, then this representation is exactly equivalent to the arrow pointing from A to an incipient A…B bond. (See below.)
4b. If the electron-flow arrow is a one-electron arrow, then this representation indicates an electron transfer from A to B. In this case, the formal charge of A increases by one, and the formal charge of B decreases by one.
5. An electron-flow arrow originating at an atom A can point to an existing bond or an incipient bond if and only if A participates in that bond or incipient bond, i.e., the sink bond or incipient bond is A-B or A…B. If the electron-flow arrow is a two-electron arrow, then the formal charge of A increases by one, and the formal charge of B decreases by one.
6. An electron-flow arrow originating at a bond A-B can point to an atom if and only if that sink atom is A or B. If the electron-flow arrow is a two-electron arrow, then, calling the sink atom A, the formal charge of A decreases by one, and the formal charge of B increases by one.
7. An electron-flow arrow originating at a bond A-B can point to another bond or incipient bond if and only if one of the atoms of the source bond is participating in the sink bond or incipient bond, i.e., the sink bond or incipient bond is B-C or B…C. If the electron-flow arrow is a two-electron arrow, then the formal charge of A increases by one, and the formal charge of C decreases by one.
8. It is improper to show an electron-flow arrow beginning at an atom with no unshared valence electrons. It is also improper to show a two-electron electron-flow arrow beginning at an atom with only one unshared valence electron.
9. It is improper to show an odd number of one-electron electron-flow arrows originating at a bond or pointing to a bond or incipient bond. (Experienced chemists will sometimes violate this rule, omitting the one-electron electron-flow arrows pointing in a particular direction. For example, for the atom abstraction reaction A· + B-C -> A-B + C·, an experienced chemist may draw only the electron-flow arrows from A to A…B and from B-C to C, and omit the one from B-C to A…B. Beginners are strongly encouraged to follow this rule.)
10. If an atom has an octet (duet for H), it is improper to show that atom accepting a new bond (or accepting an increase in the order of an existing bond) unless another bond to that atom is simultaneously broken. (Heavier elements such as P, S, etc. may break this rule.)
I should mention that the MarvinSketch applet by ChemAxon incorporates several of the structures in this scheme, for example, by disallowing an electron-flow arrow from A-B from pointing to a bond C-D.
Wonderful Bob. It is great to hear from others that are exploring arrow pushing in this way!
1. I agree that the convention quantises the electron movement into either 1 or 2 electrons. It is difficult to see how we could cope if that were not true. But juxtaposed onto rule 10, it does eventually cause problems.
10. The convention is that higher main group elements such as P,S be forced by the above rules to accept more electrons and hence violate the octet rule. In reality for those elements, the octet rule is not violated. What actually breaks down is the assumption that electrons move only in packs of two (for closed shell reactions). In something a simple as “P(V)”, the bonds in this molecule are not two-electron shared bonds, and so any arrows involved in creating a “P(V)” cannot be either.
But I think we can all see that if we allow any given arrow to deviate from being a pair, any convention using them would get very messy. I guess we have to accept such approximations in the interests of having this convention.
I would also mention that planning for the celebration of the centenary of the shared electron bond, first suggested by G. N. Lewis in 1916, is under way. Look out for a one day conference being organised by myself for March 2016 in London! We have a fantastic set of speakers already lined up, and more to come!
I generally agree with your comment on #10, although when I said, “Heavier elements such as P, S, etc. may break this rule,” I was referring to the rule about accepting an additional bond, not the octet rule.
It is important to remember that the electron-flow arrow convention relies on the approximation that all bonds are two-electron, two-atom affairs. We are all aware that this approximation works most of the time, but it breaks down in certain situations (e.g., heavy elements, transition metal complexes [try using electron-flow arrows to describe the reaction of H2O with Cl2Pd(CH2=CH2)], oligomeric organolithium and Grignard reagents). Those are the situations when the electron-flow arrow convention will likewise be insufficient. I think it’s unwise for us to try to make the electron-flow arrow convention apply to situations where the underlying assumptions on which it is built don’t apply. Having said all that, I will continue to tell my students that we can use electron-flow arrows to describe the reactions of P and S, even though we may superficially violate the octet rule in doing so.
Very useful exercise Henry, thank you. The use of a dotted line to represent a bond being formed is a a really useful convention, especially for students just beginning to learn how to draw curly arrow mechanisms. It is a shame that many textbooks instead show arrows pointing directly at the atom being “attacked”. This obscures the bond forming process, and in many cases, such as the addition of hydrogen ions to alkenes, means that the position of the incipient bond is ambiguous.
Bob’s contribution is interesting as another illustration of the value of trying to write an algorithm in clarifying the “rules” of the process. I’m engaged in a similar exercise, for an online tutorial site, and Bob’s work will be most helpful.
After a long hiatus, I have been doing a little work on an online tutor intended to help students learn to write curly arrow mechanisms (the prototype is here: http://www.ucd.ie/chem/chemint/mechanism.htm). The rules that you and Bob Grossman outlined are interesting in that context. My project does not have a rules-based analysis module at present, but I plan to add one. I have one question. In Rule 5.4 you suggest that a curly arrow can represent movement from a lone pair to a lone pair (albeit very rarely). I have been trying to think of an example of that scenario, but can’t . Can you offer an example?
Regards, Mike.
Re I have been trying to think of an example of that scenario, but can’t . Can you offer an example?
I cannot now remember whether I had an example in mind when I wrote that two years ago. Perhaps I had changes (reductions) in metal centres in mind?