In this series of posts about the electronic effects in small sulfur rings[1] I have explored increasingly large induced geometric effects. Here is the largest so far, for the compound S7I1+[2]
The calculated geometry[3] is shown below, with the crystallographic values in parentheses – the two matching very well.
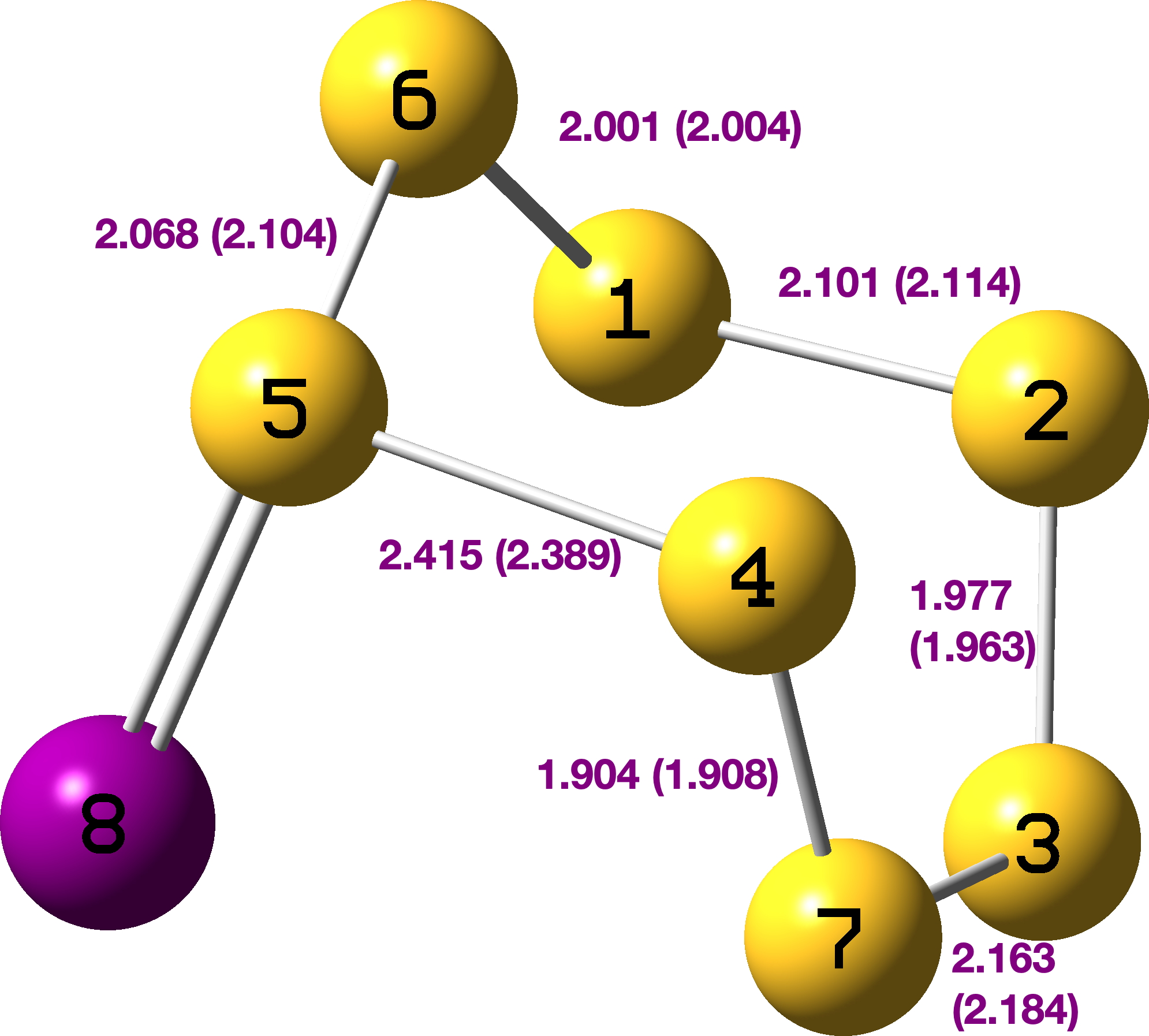
The calculated NBO7 stereoelectronic analysis identifies an especially strong donor (S7) interaction with an acceptor S4-S7, the E(2) energy being 36.9 kcal/mol. The Wiberg S4-S5 bond index is 0.512 and the S-S stretching wavenumber is ν 131. The Wiberg index for S4-S7 is 1.4618 and the S-S stretch ν 667 cm-1, matching the shortest bond.
The electronic overlap is shown below (click on image to view as a 3D model).
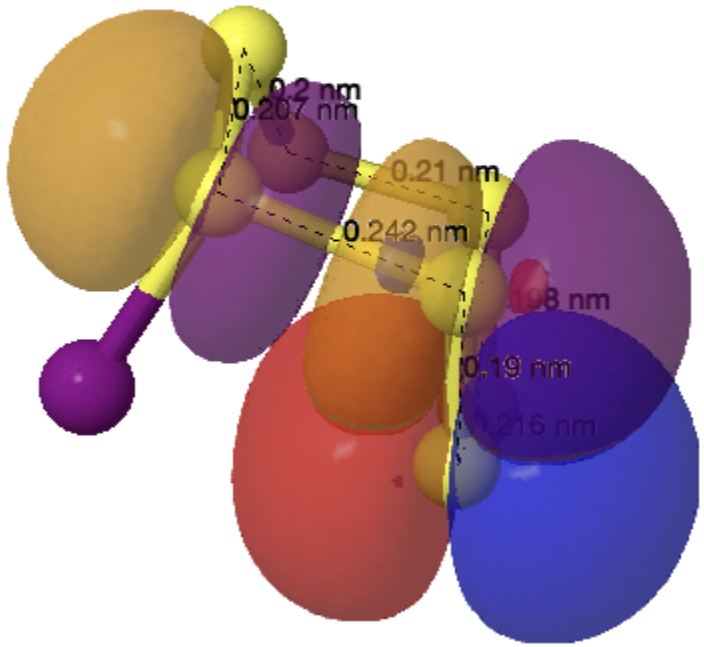
So we end with the current record for an SLp/SSσ* interaction of 36.9 kcal/mol. Who would have thought that small sulfur rings could be such fun!
References
- H. Rzepa, "5-Imino-5λ<sup>4</sup>-heptathiepane 3-oxide. More exuberent anomeric effects.", 2025. https://doi.org/10.59350/rzepa.28615
- J. Passmore, G. Sutherland, P. Taylor, T.K. Whidden, and P.S. White, "Preparations and x-ray crystal structures of iodo-cyclo-heptasulfur hexafluoroantimonate(V) and hexafluoroarsenate(V), S7ISbF6 and S7IAsF6", Inorganic Chemistry, vol. 20, pp. 3839-3845, 1981. https://doi.org/10.1021/ic50225a048
- H. Rzepa, "S7I(+) ax G = -3083.654991", 2025. https://doi.org/10.14469/hpc/15236