In an earlier post, I discussed[1] a phenomenon known as the “anomeric effect” exhibited by tetrahedral carbon compounds with four C-O bonds. Each oxygen itself bears two bonds and has two lone pairs, and either of these can align with one of three other C-O bonds to generate an anomeric effect. Here I change the central carbon to a boron to explore what happens, as indeed I promised earlier.
One can identify candidates for such molecules by a constrained search of the CSD or the Cambridge structural database, as shown below.
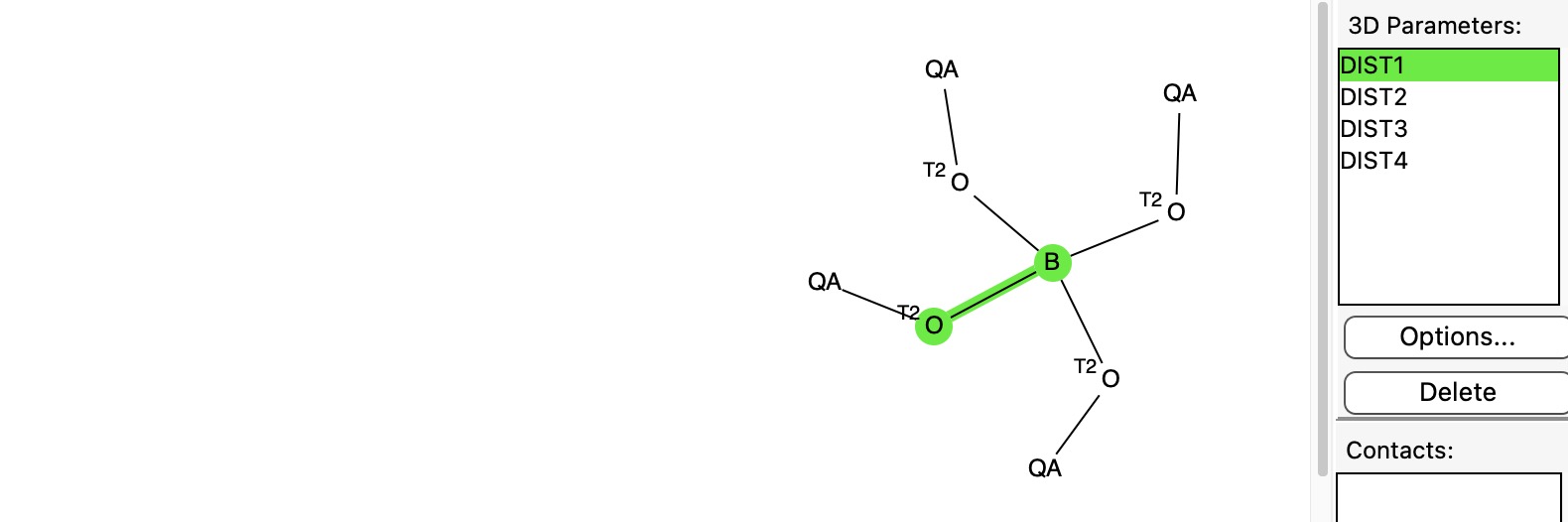
The four B-O distances for each compound matching the query are now subjected to further analysis, the greatest and least values are identified and the difference between them calculated.

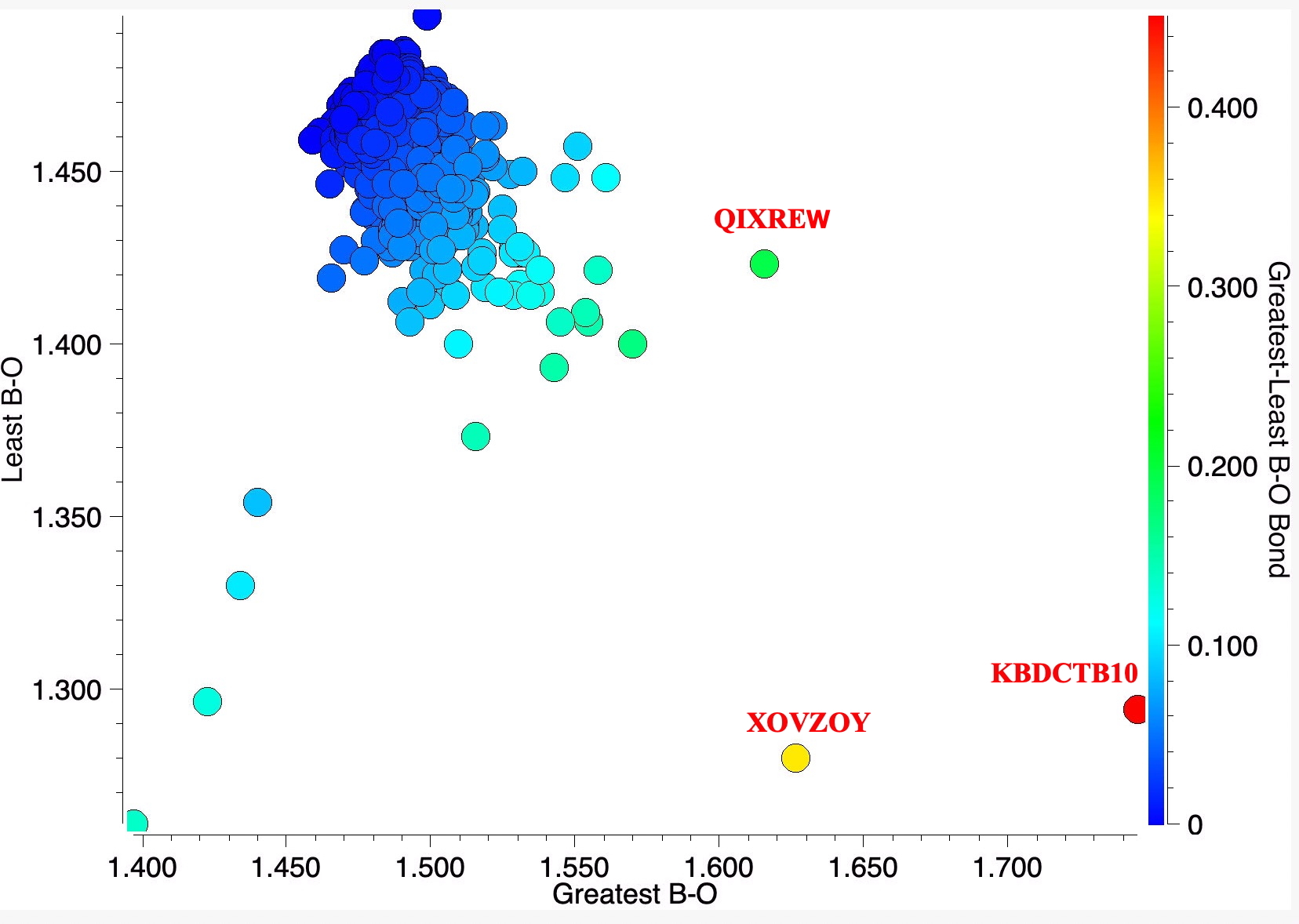
The results are shown in the diagram below. Three outliers are identified for close inspection.
Each of the three candidates is also subjected to a Gaussian calculation (MD15L/Def2-TZVPP)[2] (See DOI: 10.14469/hpc/14092)
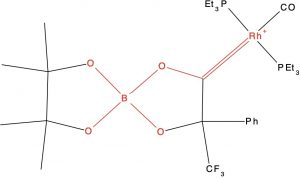
- QIXREW[3]. This molecule is overall neutral and for which ΔrB-O = 0.193Å (MN15L/Def2-TZVPP ΔrB-O = 0.175Å). The Wiberg bond indices of longest and shortest B-O bonds are 0.486 and 0.698, Δ = 0.212Å.This is significantly larger than the best example of the C-O series, for which the largest ΔrC-O = 0.074Å and 0.137 for the Wiberg index.
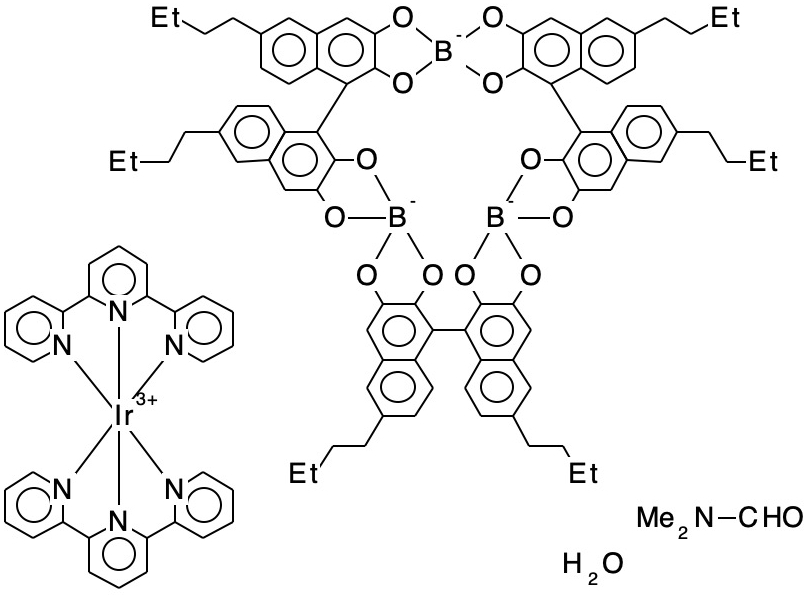
- XOVZOY[4] is a tri-anion with intercalated Ir3+ counterion. ΔrB-O = 0.347Å. A calculation on the isolated tri-anion (with a continuum water field to help emulate the crystal environment) results in the maximum B-O bond length difference of only 0.004Å, which is dramatically different from the crystal structure. This may be an example where the counter-ion is especially important for modelling structure, or it may be simply an anomalous refinement of the crystal structure.
- KBDCTB, ΔrB-O measured = 0.451Å, Calculated 0.0314Å.
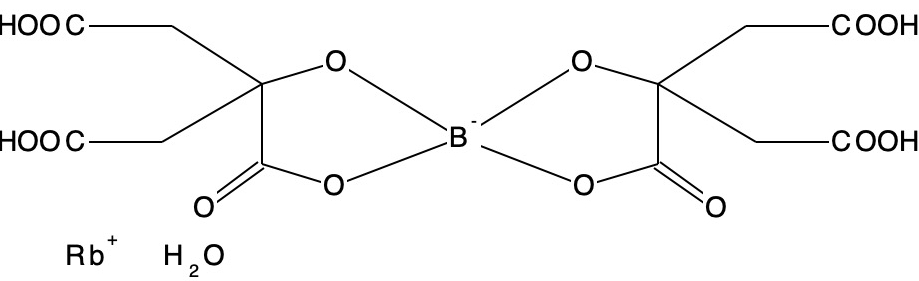
This is another structure where all may not be what it seems. This again is an anionic structure and geometry optimisation of a single molecule results in a dramatic change in the internal hydrogen bonding of the species. In the crystal structure, the carboxylic acid groups all form intermolecular hydrogen bonds. Optimized as an isolated molecule, the former are no longer possible and a big conformational change occurs to allow all four carboxylic acid groups to instead form intramolecular H-bonds. In this conformation, all four B-O bonds are essentially the same length. So this might well be an example of a large change in anomeric effects due to changes in geometry induced by hydrogen bonding.Intermolecular H-bonds Intramolecular H-bonds 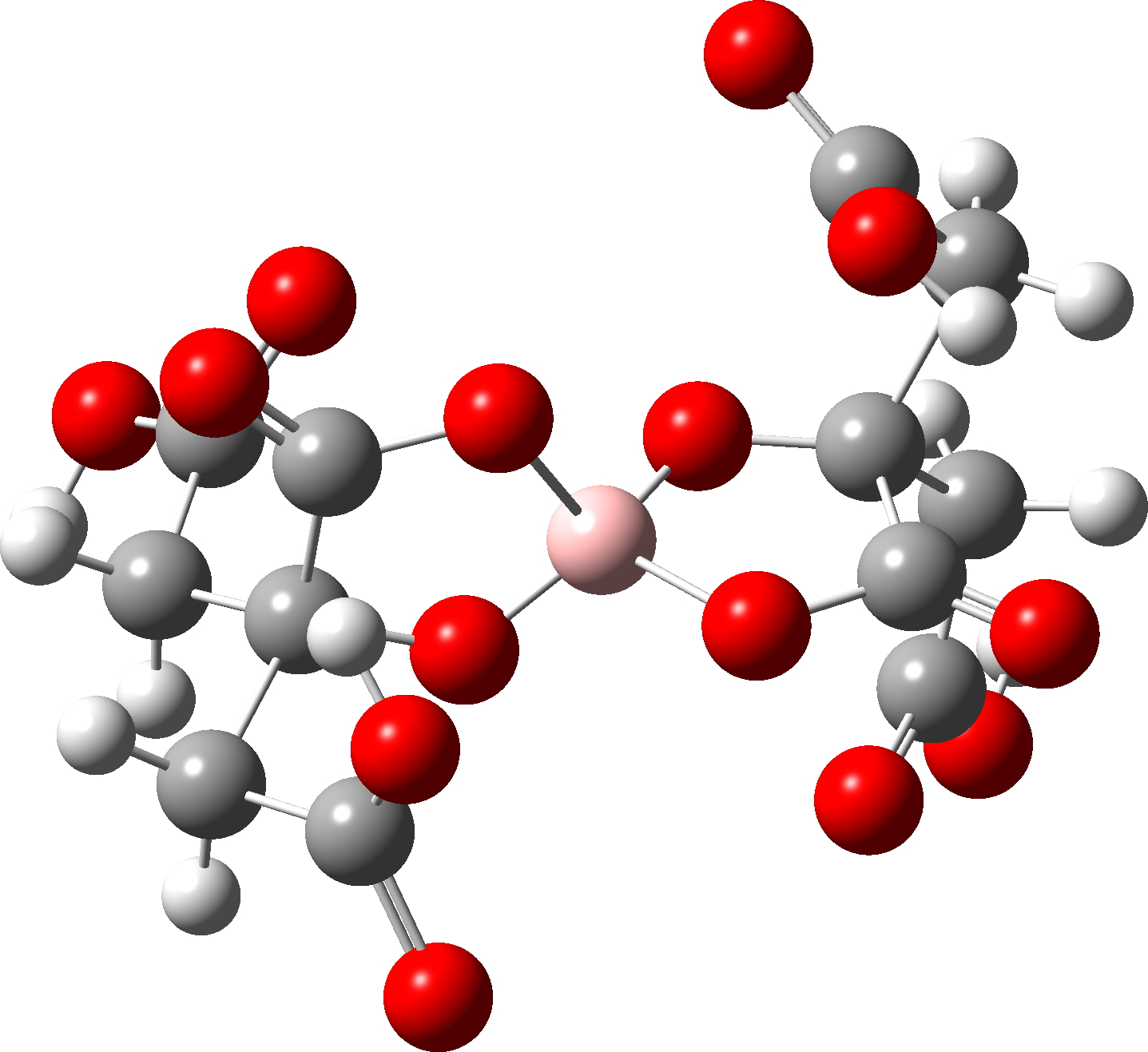

One lesson one always learns when comparing the lengths of bonds observed in crystal structures with those calculated using quantum mechanics is that they sometimes do not match well. These mis-matches can occur for various reasons; changes in hydrogen bonding, or the presence of unmodelled counterions or simply errors in the reported crystal structure. But we might suggest from this brief foray into B-O bonds that the anomeric effects found there may indeed be larger than those of their C-O counterparts.
References
- H. Rzepa, "Detecting anomeric effects in tetrahedral carbon bearing four oxygen substituents.", 2024. https://doi.org/10.59350/dfkt5-k2b20
- H. Rzepa, "Detecting anomeric effects in tetrahedral boron bearing four oxygen substituents.", 2024. https://doi.org/10.14469/hpc/14092
- S.I. Kalläne, T. Braun, B. Braun, and S. Mebs, "Versatile reactivity of a rhodium(i) boryl complex towards ketones and imines", Dalton Transactions, vol. 43, pp. 6786, 2014. https://doi.org/10.1039/c4dt00080c
- H. Danjo, K. Hirata, S. Yoshigai, I. Azumaya, and K. Yamaguchi, "Back to Back Twin Bowls of <i>D</i><sub>3</sub>-Symmetric Tris(spiroborate)s for Supramolecular Chain Structures", Journal of the American Chemical Society, vol. 131, pp. 1638-1639, 2009. https://doi.org/10.1021/ja8071435