A conjugated, (apparently) aromatic molecular trefoil might be expected to have some unusual, if not extreme properties. Here some of these are explored.
- The first is the vibrational spectrum. With 144 atoms for this molecule, it has 426 vibrational modes, but one is highlighted below. This is the mode that moves the atoms in accord with the Kekulé resonance. If real, this mode resists such alternation. The mode has a value of ~ 1310 cm-1 for benzene, although this is accepted as being lower than expected due to the phenomenon of π-distortivity (DOI: 10.1039/b911817a and also this post). The mode shown below has the value of 1650 cm-1, which is a good deal higher than for benzene. The significant coupling of the CH wagging motions with the C-C/C-N stretching (Duschinsky coupling) makes the interpretation more complex (it also occurs for benzene itself), but the Kekulé mode (there are in fact several) is surprisingly large for so many π-electrons. Perhaps the large degree of writhe noted in the previous post might have something to do with it?
Molecular trefoil: the Kekulé mode for bond alternation. Click for animation.
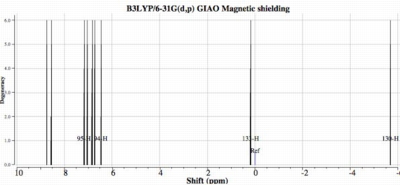
- The NICS (nucleus independent chemical shift) at the centroid of the trefoil is -16.4 ppm. This is clearly an aromatic value, and confirms our inference that the system is a 4n+2 aromatic molecule. In this example, the aromaticity is not only three-dimensional, but helical as well. The predicted 1H NMR spectrum (below) shows three regions. The upfield region (~ -5 ppm) corresponds to protons pointing directly inwards to the centre, whilst the lowfield region (~ 8ppm) corresponds to protons at the outside edge.
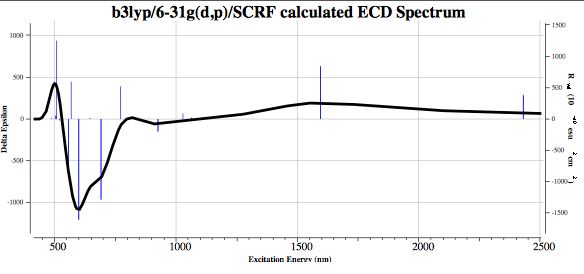
- Shown below is the calculated electronic circular dichroism (ECD) spectrum. It shows a large Cotton effect due to the chiral nature of the trefoil. The electronic transitions extend beyond ~1500nm, approaching the near infra-red. The phase of the Cotton effect at ~600nm calculated for the chiral isomer shown in the 3D model above would certainly suffice to assign the absolute configuration of the system should the experimental spectrum be measurable.
The spectrum above shows maximum absorption at ~600nm, which means optical rotation at the sodium D-line (589 nm) cannot be measured (light has to get through to measure its rotation). However, the region of 880nm (the highest value available on commercial spectrometers) is reasonably transparent for such measurement. Calculations may not be much help, since the linear CPHF equations appear unstable. Thus [α]880 shows an enormous dependence on the precise DFT method chosen to compute it (~ +8763°@CAM-B3LYP but the very different -59898°@B3LYP).
Henry Rzepa. Chemistry with a super-twist: A molecular trefoil knot, part 2.. . 2010-06-02. URL:http://www.ch.ic.ac.uk/rzepa/blog/?p=2084. Accessed: 2010-06-02. (Archived by WebCite® at http://www.webcitation.org/5qC4NiFsM)
Tags: animation, chemical shift, chiroptical, Henry Rzepa, Interesting chemistry
Will you submit the (calculated) NMR spectrum to the NMRShiftDB? That way, your theoretical predictions will go into the main databases for future reference.
To answer Egon, it depends on the workflow available. The raw NMR data in Gaussian log files needs a fair bit of processing (the TMS reference needs computing, or looking up from somewhere). I use Gaussview to display a more processed spectrum (it has a pre-built reference look up) but this program is not good at exporting in a sensible format.
So what mash-ups could be applied to automate the process? If it can be scripted, then we could add the workflow to our digital repository. But there is another issue; QA. Is the DFT method suitable? Is the basis set good enough? Is the solvation model suitable (or indeed has it been applied?). Should spin-spin couplings be computed (for 1H)? It is not entirely trivial, but if it were done, it would correspond to what Peter Murray-Rust once called the World-Wide molecular matrix, whereby a molecule is injected, a variety of properties computed, and the results outputted in discoverable manner.
[…] and aromaticity. One might ask whether any of the preceding experiments might relate to the molecular trefoil I described in another […]