
|
Steroids are naturally-occurring substances of both plant & animal origin which have considerable medical importance -
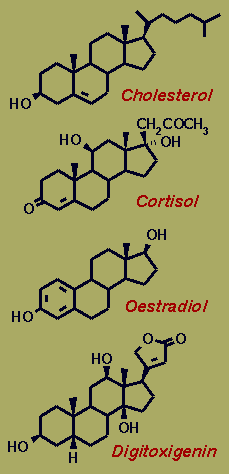
Most mammalian steroids are derived from Cholesterol. Despite a
recent bad press, it is in fact an essential
cellular component in mammals (the average human adult carries about 250g) and
undergoes further biosynthetic transformations - part of the C17-sidechain is
removed to give glucocorticoids, such as cortisol, and complete removal leads to
the sex hormones testosterone & oestradiol.
Much of early steroid chemistry was developed on cholesterol - readily available
from gallstones! Once the structure of the ring system had been solved - a
fascinating chemical odyssey - the basic steroid skeleton proved particularly
valuable for the development of theories of both reaction kinetics and
reaction mechanism, providing a rigid framework upon which the effects of
conformation and configuration could be explored.