
 1
2
3
4
5
1
2
3
4
5

Experiment 8, page 1

Department ofChemistry, Imperial College
Third YearAdvanced Practical Organic Chemistry


EXPERIMENT 8:
TRANSFER HYDROGENATION IN THE REDUCTION OF KETONES
Aims of the experiment
To prepare NiCl2(PPh3)2and examine its use as a transfer hydrogenation catalyst for the reduction
of ketones to alcohols, using iso-propanol as the hydride source.
Techniques used/learned:
Preparation of transition metal complexes; recrystallisation.
Introduction
Hydrogenation is a commonly used technique in organic chemistry.1The combination of gaseous
hydrogen in conjunction with a heterogeneous transition metal catalyst has been used to effect
many useful reductions, egalkenes, alkynes and aromatics to alkanes; nitro, nitrile, imine and oxime
groups to amines; and cleavage of benzylic ethers and amines (useful in protecting group
chemistry). The use of modified catalysts (such as the partially poisoned Lindlar palladium
catalyst) can allow selective reactions or partial reductions, egof alkynes to Z-alkenes.
More recently, well defined transition metal complexes such as chloro(tristriphenylphosphine)
rhodium(I) (Wilkinson's catalyst, discovered at Imperial College)2have found wide application as
homogeneous catalysts. Often these are more efficient than their heterogeneous counterparts, since
the active hydrogenation species and the substrate for reduction are in the same (liquid) phase. The
use of such complexes has opened the way for the design of efficient asymmetric catalysts, using
chiral ligands in the metal complex (often phosphines). In this fashion, one can enantioselectively
reduce a range of functional groups (egalkenes, ketones, imines and enamides - the latter two are
useful for the synthesis of amino acids). This is an important industrial process: for example, it is
used in the asymmetric commercial synthesis of the important anti-Parkinson's agent L-DOPA.3
The area of transfer hydrogenation is of growing interest. Although hydrogen is a relatively cheap
reducing agent, it does pose issues of handling and containment. For example, reactions need to be
done with the rigorous exclusion of air (to prevent possible explosions), and often need to be
carried out at elevated pressures to overcome phase transfer. The use of alternative, readily soluble
reducing agents to transfer hydrogen to a metal catalyst is therefore of interest. Typical agents
include cyclohexadiene (which loses hydrogen to generate benzene), formate salts (effectively
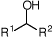
generates H-and CO2), and alcohols (which are oxidised to ketones).
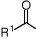
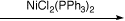
R2i-PrOH
In this experiment, you will prepare an achiral homogeneous hydrogenation catalyst (NiCl2(PPh3)2)
and examine its use as a transfer hydrogenation catalyst for ketones.



 1
2
3
4
5
1
2
3
4
5

![]()
![]()
![]()
![]()
![]()
![]()