
![]() EXPERIMENT 6:
EXPERIMENT 6:
OZONOLYSIS OF AN OLEFIN
To oxidatively cleave an olefin and trap the resulting carbonyl fragments as their 2,4-
dinitrophenylhydrazone derivatives.
Use of an ozoniser; steam distillation; column chromatography; recrystallisation.
Ozone readily attacks ethylenic linkages and from the products, carbonyl compounds can be
obtained.1![]() The process results in separation of the carbon atoms originally joined by the double
The process results in separation of the carbon atoms originally joined by the double
bond. The identities and yields of carbonyl products provide information on the positions of the
double bonds in the olefin. Hence ozonolysis is frequently used in structure determination as well
as for synthetic purposes.
The work of Criegee2![]() indicates that the preferred mode of addition to most olefins is via
indicates that the preferred mode of addition to most olefins is via![]() a
a
molozonide 1. In some cases these can be isolated; reduction at low temperatures can then be made
to produce glycols. In general however, the molozonide 1![]() rapidly decomposes to carbonyl and
rapidly decomposes to carbonyl and
peroxy (carbonyl oxide) products. These rapidly recombine in non-polar solvents to give an
ozonide 2. Occasionally, a polymer results or the carbonyl oxide may react with polar solvent.
Hydrolysis or (preferably) reduction of the the ozonide 2![]() liberates the two carbonyl compounds.
liberates the two carbonyl compounds.
hydrolysis produces carboxylic acid instead of aldehyde. It is therefore preferable to use the
reductive work up to reduce the peroxy link. As reducing agents, Zn/AcOH, Me2S, Ph3P, Me3P,
and (NC)2C=C(CN)2![]() have all been used (see Scheme 1): catalytic hydrogenation is hazardous and
have all been used (see Scheme 1): catalytic hydrogenation is hazardous and
not recommended. Conversely, treatment of the ozonide with peracids gives ketones and
carboxylic acids directly.

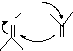
![]() O
O
O
![]() O–+
O–+
![]() +
+
![]() O
O
O
![]() 3
3
![]() O
O
O
![]() 4
4
![]() 2
2
![]() 4
4
![]() 1
1
![]() 1
1
![]() 2
2
![]() 3
3
![]() 4
4