Email Discussion:
Re: Poster 26 Donald Craig : Template-Directed Intramolecular
Tom McCarthy
Email Discussion:
Re: questions on poster 26 from Dr Tom McCarthy
d.craig@ic.ac.uk
Template-Directed Intramolecular C-Glycosidation
Donald Craig,* Andrew H. Payne, Mark W. Pennington, Jason P. Tierney
Department of Chemistry, Imperial College of Science, Technology and Medicine
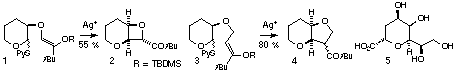
We have been developing strategies for the stereoselective synthesis of
bicyclic and monocyclic C-glycosides by cationic cyclisation reactions.
Thus, bicyclic ketooxetanes 2 and ketooxolanes 4 may be prepared
by silver triflate-mediated closure of substrates 1 and 3.[1],2 We have successfully applied
this chemistry to the enantioselective synthesis of the potent KDO inhibitor
2,3-deoxy-D-manno-2-octulopyranosate 5.[3]
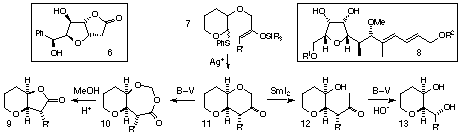
We are now seeking to exploit this strategy to access stereochemically more
complex bicyclo[4.4.0] systems, and to explore a range of derivatisation
reactions of these materials. Thus, substrate 7 has been prepared, and
we are looking at its silver (I)-induced cyclisation. Baeyer-Villiger (B-V)
oxidation of the ring-closed product 11 is expected to yield acetals
10 and thence lactones 9. Subjection of 11 to reductive
tether cleavage would give methyl ketones 12, from which carbinols
13 could be made by Baeyer-Villiger oxidation followed by hydrolysis.
It is intended to apply these sequences to the synthesis of the antitumour
agent goniofufurone 64 and the central sub-unit 8 of
the antibiotic efrotomycin.[5]
The paper is presented as a collection of slides, click on the hyperlinks below to receive the pictures:
- Introduction
- Strategy
- Prototype substrates
- Sythesis of prototype substrates
- Cyclisation Reactions
- Cyclisation of a more substituted substrate
- Oxetanes are accessible using this strategy!
- Synthesis of 5-membered bicyclic C-glycosides
- The separate anomers of thioglycoside show different cyclisation selectivities
- Application to bioactive compound synthesis
- Retrosynthetic analysis of 2-deoxyKDO
- 2-DeoxyKDO synthesis: assembly of the pyran sigma-framework
- Pyran construction and adjustment of stereochemistry
- Glycal formation and attachment of a cinnamyl side-chain
- Cyclisation os the cinnamyl ether and elaboration of the dihydropyran
- Completion of the synthesis of 2-deoxyKDO
- Acknowledgements
References
[1] Craig, D.; Munasinghe, V. R. N.
J. Chem. Soc., Chem. Commun. 1993, 901.
2 Craig, D.; Pennington, M. W.; Warner, P. Tetrahedron Lett.
1993, 34, 8539.
[3] Craig, D.; Pennington, M. W.; Warner, P., manuscript in
preparation.
[4] See: Fang, X.-P.; Anderson, J. E.; Chang, C.-J.; Fanwick, P. E.;
McLaughlin, J. L. J. Chem. Soc., Perkin Trans. 1, 1990, 1665.
[5] Dolle, R. E.; Nicolaou, K. C. J. Am. Chem. Soc.
1985, 107, 1691, 1695, and references therein.