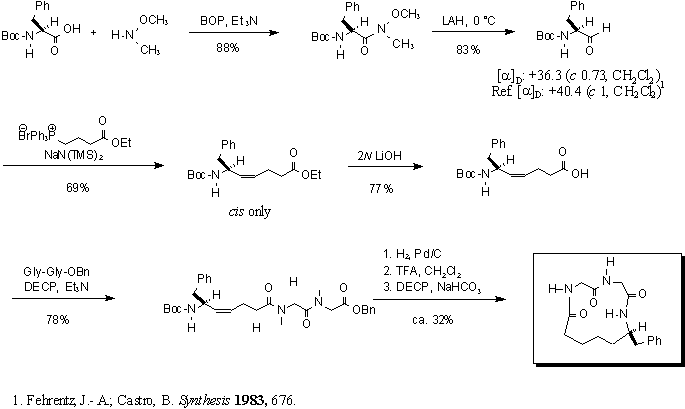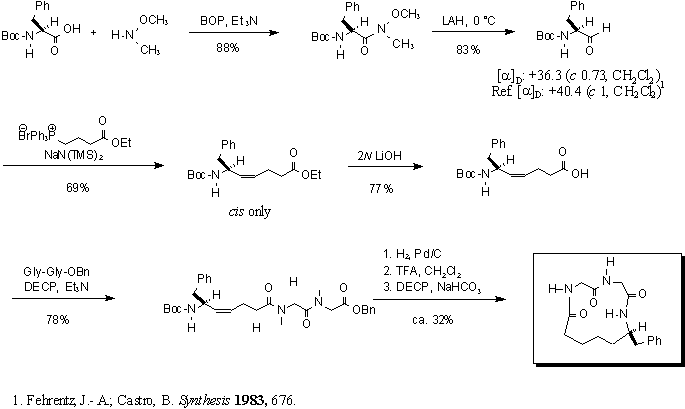Synthesis of 1,4,7-Triaza-8(S)-benzyl-3,6,13-trione
The key steps in this scheme include the conversion
of L-Phenylalanine to the corresponding Weinreb amide, which is easily
reduced to L-Phenylalanal using lithium aluminum hydride. The aldehyde
is subject to Wittig conditions to extend the carbon chain by four carbons.
The resultant ester is hydrolyzed and coupled to the C-terminus protected
Gly-Gly peptide. The tripeptide undergoes hydrogenation followed
by treatment with trifluoroacetic acid to deprotect the C- and N-termini,
respectively, before it is cyclized to afford the desired macrocycle.
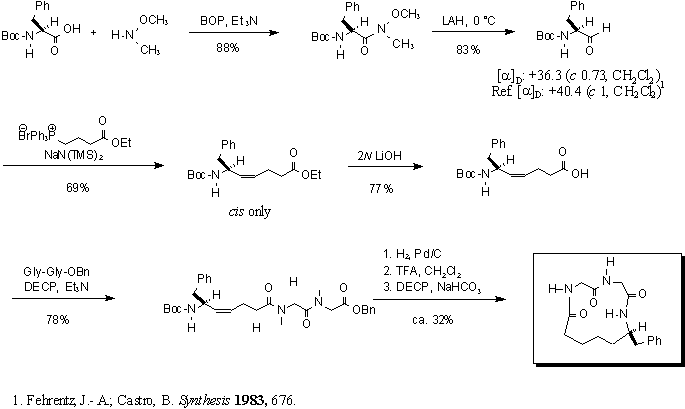
Back to Index
Synthesis of the 3- and 5-Benzyl-Aca Linkers